0998
Improved Speed and Image Quality for Imaging of Liver Lesions with Auto-calibrated Wave Encoded Variable Density Single-Shot Fast Spin Echo.1Radiology, Stanford University, Stanford, CA, United States, 2Electrical Engineering and Radiology, Stanford University, Stanford, CA, United States, 3Global MR Applications and Workflow, GE Healthcare, Menlo Park, CA, United States
Synopsis
Abdominal T2-weighted imaging is conventionally lengthy, but single shot approaches significantly improve current acquisition times. For single shot fast spin echo (SSFSE), axial imaging speed and sharpness are constrained by limited parallel imaging acceleration. Here, SSFSE technique with wave encoding and variable-density sampling (wSSFSE) was developed to enable higher accelerations and improve overall image quality. The purpose of this study is to assess image quality, delineation of anatomical structures, lesion conspicuity, and speed improvements with wSSFSE.
Introduction
T2-weighted imaging is a critical component of body MR protocols. Standard fast spin echo (FSE) can produce diagnostic quality T2 weighted images but typically takes several minutes1. In this time, image degradation occurs due to voluntary patient motion, respiratory artifact, and bowel motion. These shortcomings are mitigated by the use of single-shot (SSFSE) methods, but typically with less SNR and image sharpness in comparison to the standard FSE approach2.
Recently, a modified SSFSE approach with variable refocusing flip angles and full-Fourier acquisition was described and shown to lead to higher SNR and image sharpness3-4. However, parallel imaging acceleration is limited to approximately 2-fold for axial imaging due to limited number of sensitive coils in this direction, and thus T2-decay related blurring remains. Wave encoding5 and its analogs6 can leverage coil sensitivities in the readout direction to increase parallel imaging capability, and can be combined with compressed sensing (CS)7. Here we develop and clinically evaluate a wave-encoded single shot technique combined with a CS reconstruction in the clinical setting of neuroendocrine tumor (NET) metastasis to the liver.
Methods
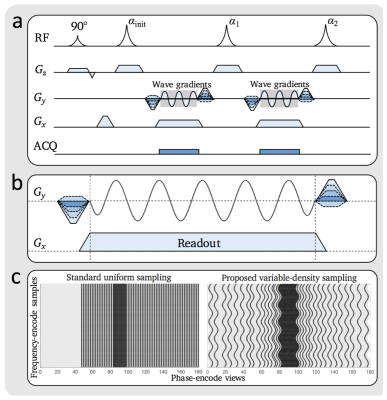
A sinusoidal wave-encoding gradient and variable density sampling was added to a SSFSE sequence with variable refocusing flip angles. Acquisition parameters: 5 mm slice thickness, 0.8 phase FOV, ± 244.1 Hz/pixel effective bandwidth, 320 x 224 matrix, 5 cycles, 10 mT/m wave-encoding gradient amplitude, and 3.5-fold acceleration. This sequence (Fig. 1), as further described8, was included in a liver MRI protocol with gadoxetic acid (Eovist) contrast administration.
With IRB approval and waived consent, we retrospectively identified all patients from 9/21/2017 - 10/21/2017 who had a 3T MRI with a 32-channel torso phased-array and Eovist for evaluation of NET metastases. A standard respiratory-triggered FSE-T2-weighted scan (15 echo-train-length, 5 mm slice thickness, ±284.4 Hz/pixel bandwidth, 320 x 224 matrix, no acceleration,), a conventional SSFSE scan (5 mm slice-thickness, 0.8 phase FOV, ±260.4 Hz/pixel bandwidth, 320 x 224 matrix, 2 fold-acceleration), and a wave-encoded single shot (wSSFSE) were all acquired. All three T2-weighted sequences were fat-suppressed. High-resolution delayed hepatobiliary phase navigated 3D SPGR images (2.2 mm slice thickness, 0.8 phase FOV, ±284.4 Hz/pixel bandwidth, 320 x 320 matrix, flip-angle 25 degrees) with intermittent fat suppression served as a gold standard for presence of lesions.
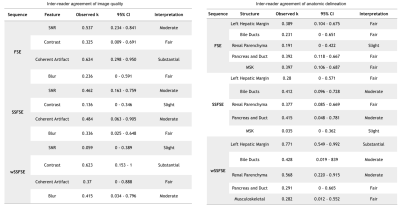
Two radiologists independently assessed the FSE, SSFSE and wSSFSE images for image quality (subjective SNR, contrast, aliasing/motion artifacts, blurring) and anatomic delineation of the liver, bile ducts, kidneys, pancreas, and musculoskeletal structures on a five point scale.
For each subject, the delayed hepatobiliary phase images were used to identify a true lesion. Then for each such lesion, lesion conspicuity was scored in randomized blinded fashion for FSE, SSFSE and wSSFSE. Subsequently, side-by-side blinded evaluations of lesion conspicuity was performed on a 7 point scale (-3 to 3; 0 implying equivalence).
Cohen’s kappa was used to evaluate inter-reader agreement. One way analysis of variance (ANOVA) tests were performed for paired, nonparametric analyses to determine if wSSFSE was comparable to standard FSE and SSFSE. Bonferroni-Holm correction was used for multiple comparisons.
Results
The final cohort of 20 patients had an average age of 53 ± 12 years, with 11 males. Repetition time averaged 646 ms for SSFSE versus 535 ms for wSSFSE. This resulted in breath-holds that were on average 17% shorter for wSSFSE.
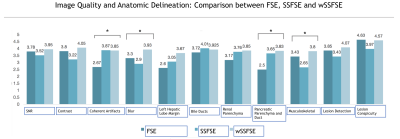
Inter-reader agreement (Table 1) was substantial for both general image quality metrics and anatomic delineation. Compared to SSFSE, wSSFSE demonstrated significantly less subjective noise and greater sharpness while maintaining comparable contrast, lesion conspicuity, and artifacts (Fig. 2). Overall, compared to FSE, wSSFSE had no statistically significant difference in image quality or anatomic delineation.
Regarding lesion conspicuity, no difference was noted between wSSFSE and FSE. More importantly, frequency of lesion detection did not differ by technique; every case of metastasis with adequate conspicuity on FSE was also demonstrated to have equivalent conspicuity on wSSFSE. This implies comparability of wSSFSE and FSE for true lesion delineation.
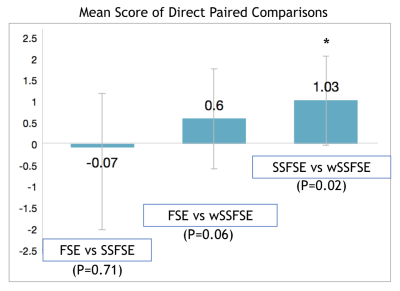
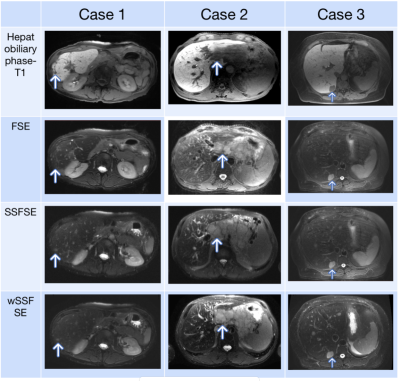
Side-by-side evaluations revealed a preference for wSSFSE over SSFSE (Fig. 3). No statistically significant preference was observed with FSE compared independently to SSFSE or wSSFSE. Representative example images are shown in Fig. 4.
Discussion
We demonstrate that wSSFSE can significantly improve image sharpness, spatial resolution, and subjective SNR compared to SSFSE while shortening acquisition time compared to standard SSFSE and FSE. Delineation of some structures was improved with wSSFSE compared to SSFSE and FSE. Lesion conspicuity was not statistically different between the methods. A current limitation of the wSSFSE method is a long image reconstruction time.Conclusion
Variable density wave-encoded single shot fast spin echo enables fast axial abdominal T2 weighted imaging with image quality and lesion conspicuity comparable or better than FSE. This can dramatically speed abdominal MRI exams.Acknowledgements
This work was supported by NIBIB R01EB009690 and GE Healthcare.References
1. Semelka RC, Kelekis NL, Thomasson D, Brown MA, Laub GA. HASTE MR imaging: description of technique and preliminary results in the abdomen. J Magn Reson Imaging 1996;6:698–699.
2. Loening AM, Litwiller DV, Saranathan M, Vasanawala SS. Increased speed and image quality for pelvic single-shot fast spin-echo imaging with variable refocusing flip angles and full-Fourier acquisition. Radiology 2017;282:561–568.
3. Loening AM, Saranathan M, Ruangwattanapaisarn N, Litwiller DV, Shimakawa A, Vasanawala SS. Increased speed and image quality in single-shot fast spin echo imaging via variable refocusing flip angles. J Magn Reson Imaging 2015;42:1747–1758.
4. Bilgic B, Gagoski BA, Cauley SF, Fan AP, Polimeni JR, Grant PE, Wald LL, Setsompop K. Wave-CAIPI for Highly Accelerated 3D Imaging. Magn Reson Med 2015. 73(6): 2152-62.
5. Moriguchi H, Duerk JL. Bunched Phase Encoding (BPE): A New Fast Data Acquisition Method in MRI. Magn Reson Med 2006. 55(3):633-648.
6. Curtis AT, Bilgic B, Setsompop K, Menon RS, Anand CK. Wave-CS: Combining wave encoding and compressed sensing. Proceedings of ISMRM 2015, 82.
7. Chen F, Taviani V, Tamir JI, Cheng JY, Zhang T, Song Q, Hargreaves BA, Pauly JM, Vasanawala SS. Self‐Calibrating Wave‐Encoded Variable‐Density Single‐Shot Fast Spin Echo Imaging. Journal of Magnetic Resonance Imaging. 2017 Sep 14.
8. Griswold MA, Jakob PM, Chen Q, et al. Resolution enhancement in single-shot imaging using simultaneous acquisition of spatial harmon- ics (SMASH). Magn Reson Med 1999;41:1236–1245.
9. Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocali- brating partially parallel acquisitions (GRAPPA). Magn Reson Med 2002;47:1202–1210.
10. Gagoski BA, Bilgic B, Eichner C, et al. RARE/turbo spin echo imaging with Simultaneous Multislice Wave-CAIPI. Magn Reson Med 2015;73: 929–938.
Figures