0996
L-Carnitine Shows Beneficial Effects on Cardiac Metabolism and Function: A Hyperpolarized MRS and Langendorff Perfusion Study1University of Oxford, Oxford, United Kingdom
Synopsis
L-carnitine acts as a buffer of acetyl-CoA units in the mitochondria, as well as facilitating transport of fatty acids. In addition, L-carnitine levels are decreased in the diabetic heart. The purpose of this study was to investigate the effect of L-carnitine supplementation on cardiac function and metabolism in the diabetic rat heart. We show that daily injections of L-carnitine can alter cardiac metabolism in the in-vivo diabetic rat heart, and can improve functional recovery as well as fatty acid oxidation rates post ischemia. Such studies allow a better understanding of the interactions between metabolism and function in the diabetic heart and may provide new insight into novel therapeutics.
Introduction
Diabetes increases the incidence of myocardial infarction and heart failure, which are leading causes of mortality in diabetic patients [1]. Improving our understanding of the metabolic changes that occur in the diabetic heart may be key to understanding the pathophysiology of the cardiac effects of diabetes. Several studies have shown that L-carnitine levels are decreased in the diseased state and that L-carnitine supplementation protects cardiac function in disease [2,3]. L-carnitine acts as a transporter for fatty acids as well as acting, via acetyl-carnitine, as a buffer of excess acetyl-CoA units in the mitochondria. It may therefore have multiple effects on the balance of the energy sources used in the diabetic heart [4]. The purpose of this study was to explore the metabolic and functional effects of daily L-carnitine supplementation on the diabetic rodent heart.Methods
49 male Wistar rats (~200g) were split into two groups; rats injected with streptozotocin (STZ, 55 mg/Kg) to induce a model of type 1 diabetes and rats injected with citrate buffer (CTR) to act as controls. Two weeks after injection, daily intra-peritoneal injections of either 600μl of saline or L-carnitine (3g/kg/day) diluted in saline were initiated and continued for 3 weeks. Following treatment, ECG gated 13C MR pulse-acquire cardiac spectra were acquired over 60s following an injection of hyperpolarized [1-13C]pyruvate (repetition time 1s; excitation flip angle 15°; sweep width 13,021Hz; acquired points 2,048; frequency centred on the C1 pyruvate resonance). Acquired spectra were summed over 30s from the first appearance of pyruvate and analysed with JMRUI [5] for metabolic assessment of the heart. In addition, eight-ten short-axis slices (slice thickness:1.6mm, matrix size:128×128, TE/TR:1.67/4.6ms, flip angle:15°, number of averages:4) were acquired with a CINE-FLASH sequence and analysed with ImageJ for assessment of cardiac function. A subsequent cohort was used for Langendorff heart perfusion and subjected to a protocol of ischemia-reperfusion as described by Heather et al. [6] with fatty acid oxidation measured as described by Lopaschuk et al. [7].Results
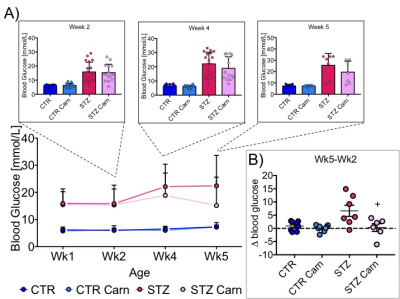
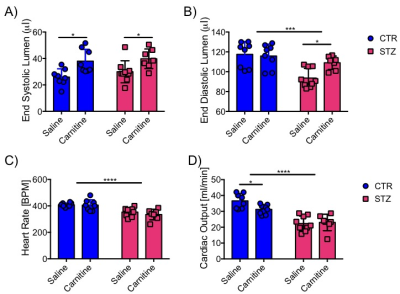
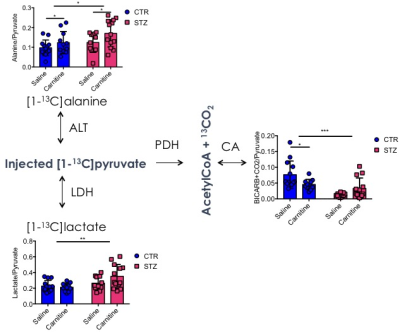
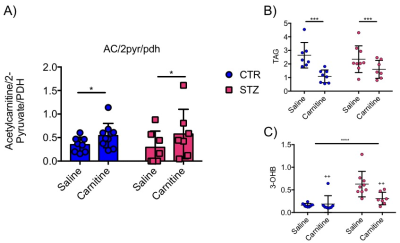
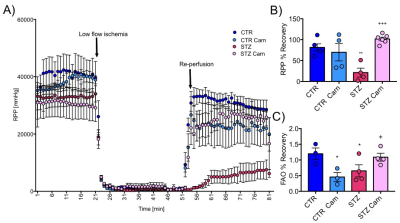
Blood glucose levels were elevated in the diabetic groups (Fig. 2A) with the saline treated STZ group showing a significant and progressive increase in hyperglycaemia over the three weeks of treatment (Fig. 2B). In contrast, whilst the L-carnitine treated STZ group also showed elevated blood glucose at 2 weeks, there was no further increase over the duration of the treatment (Fig. 2B). Average left ventricular mass, stroke volume, ejection fraction, end diastolic lumen (EDL), heart rate and cardiac output were all depressed in the two STZ groups (Fig. 2), however, the L-carnitine treated diabetics showed an improvement in EDL (Fig. 2B). Incorporation of the 13C label into alanine and lactate was significantly increased in the diabetic groups, whilst L-carnitine treatment also significantly increased incorporation of 13C in the alanine pool (Fig. 3). Flux through pyruvate dehydrogenase (PDH) was significantly reduced in the saline and L-carnitine treated STZ animals compared to the control animals (Fig. 3). L-carnitine treatment increased acetylcarnitine levels (Fig. 4A) and decreased triacylglycerides (TAG) in both control and STZ treated animals (Fig. 4B), furthermore L-carnitine treatment normalised β-hydroxybutyrate (3-OHB) in the STZ animals to control levels (Fig. 4C). The L-carnitine STZ hearts recovered significantly better post low-flow ischemia compared to the saline treated STZ group for both the rate pressure product (RPP) (Fig. 5B) and fatty acid oxidation (FAO) rates (Fig. 5C).Discussion and Conclusion
This study shows beneficial effects of L-carnitine supplementation in diabetic animals. Despite demonstrating no differences in cardiac function, L-carnitine treatment slowed the progression of hyperglycaemia and normalised fatty acid oxidation recovery as well as functional recovery post ischemia. This study confirms a significant increase of acetylcarnitine in the heart following daily injections and, when treated with L-carnitine, a significant decrease in β–hydroxybutyrate levels. Previous studies have shown a beneficial effect of L-carnitine treatment on cardiac function in the perfused heart [2,8]. In this study we confirm this finding, and also show a potential recovery in diastolic relaxation. It is possible that cardiac function may be improved over an even longer period of treatment as Rodrigues et al. [2] suggest. L-carnitine supplementation leads to alterations in metabolism in the in-vivo heart and can restore oxidation rates and function after a heart attack. It has shown multiple beneficial effects in the diabetic heart affirming that the balance of energy sources the heart is using is of critical importance. The use of L-carnitine supplementation in the diabetic heart appears beneficial but needs further investigation.Acknowledgements
No acknowledgement found.References
[1] Aaron
M. Secrest, Raynard E. Washington and Trevor J. Orchard "Mortality in Type
1 Diabetes" Chapter 35, Oct 2014.
[2] B. Rodrigues, H. Xiang, and J. H.
Mcneill, “C 1358,” vol. 37, no. October, 1988.
[3] A. J. Liedtke, T. C. Vary, S. H.
Nellis, and C. W. Fultz, “Properties of L-carnitine Incorporation in Working
Swine Hearts Effects of Coronary Flow , Ischemia , and Excess Fatty Acids,”
1981.
[4] T. Doenst, T. D. Nguyen, and E. D.
Abel, “Cardiac metabolism in heart failure: implications beyond ATP
production.,” Circ. Res., vol. 113, no. 6, pp. 709–24, Aug. 2013.
[5] Vanhamme L, van
den Boogaart A, Van Huffel S. Improved method for accurate and efficient
quantification of MRS data with use of prior knowledge. J Magn Reson
1997;129:35-43
[6] Heather
L, Pates K, Atherton H, Cole M. Ball D et al. " Differential translocation
of the fatty acid trasnport, FAT/CD36, and the glucose trasnporter, GLUTr,
coordinates changes in cardiac substrate metbaolism during ischemia and
reperfusion" Circ. 6, 2013,1058-66.
[7] G.
Lopaschuk and R. Barr "measurements of fatty acid and carbohydrate
metabolism in the working rat heart", Molecular and Cellular Biochemistry
172, 137-147, 1997.
[8] J.
frochlich, D. Seccombre, P. Hahn and P. dodel 1978 27(5), 555-561.
Figures