0991
Glycogen Synthesis Mapping Using In Vivo Deuterium Metabolic Imaging (DMI)1Department of Radiology and Biomedical Imaging, Yale University, New Haven, CT, United States, 2Department of Psychiatry, Yale University, New Haven, CT, United States, 3Department of Biomedical Engineering, Yale University, New Haven, CT, United States
Synopsis
Deuterium Metabolic Imaging (DMI) is a novel approach providing high spatial resolution metabolic data from both animal models and human subjects. DMI relies on 2H MRSI in combination with administration of 2H-labeled substrates. We show how DMI combined with administration of [6,6’-2H2]-glucose can image liver glycogen synthesis in rats and human subjects. In rats at 11.7T, DMI revealed differences in liver glycogen synthesis between glucose administrations through an intravenous and intraperitoneal route. At 4T, we showed that DMI is feasible in humans and could detect labeling of liver glycogen after oral intake of [6,6’-2H2]-glucose.
Introduction
Liver glycogen synthesis plays a key role in whole body glucose homeostasis and is altered by metabolic disorders such as type 2 diabetes and nonalcoholic fatty liver disease (NAFLD) (1). Liver glycogen synthesis has been measured previously in vivo with 13C MRS and 13C-labeled glucose infusion (2,3). However, 13C MRS suffers from low sensitivity, can be technically challenging and has only provided glycogen synthesis data from large, single voxels. Here we introduce deuterium metabolic imaging (DMI), which relies on 2H MRSI combined with administration of a 2H-labeled substrate, for spatially mapping of liver glycogen synthesis in vivo in rats and humans.Methods
Animals and human studies were performed on 11.74T and 4T magnets, both interfaced to Bruker Avance III HD spectrometers. Rat liver studies were performed with a proton volume coil for MRI and shimming, and a deuterium volume coil for 2H MRS(I), mounted inside the 1H coil. For human liver studies we used a custom-built 1H-2H probe consisting of a 2H surface coil with a pair of elliptical 1H coils arrayed in quadrature for MRI and shimming. 3D 2H MRSI using spherical k-space encoding was performed without respiratory gating, using a pulse-acquire sequence extended with 3D phase-encoding gradients. In rats, signal excitation was achieved by selecting a 25 mm thick transverse slab to minimize artifacts originating from the heart. DMI in rat liver was performed at a 4x4x4mm3=64 µL nominal spatial resolution in circa 25 min (TR=400ms, averages=4). Human liver DMI data were acquired with a 90° rectangular RF pulse, at a nominal 25x25x25mm3=15.6 mL spatial resolution. With TR=333ms and 8 averages a steady-state DMI acquisition took circa 25 min.
After an overnight fast Fischer 344 rats were infused for 120 minutes with [6,6’-2H2]-glucose (1M, 1.5g/kg) either via an intravenous (IV, n=3) line in the femoral vein or intraperitoneal (IP, n=3) infusion. Human DMI data acquisition (n=2) started ~120 minutes after an oral dose of [6,6’-2H2]-glucose (0.75g/kg), dissolved in 200mL of water.
All 1H MRI and 2H DMI data were processed in NMRWizard, a home-written graphical user interface in Matlab 8.3. DMI processing included linear prediction of the missing time domain points due to the phase encoding gradients and 5 Hz line broadening followed by 4D Fourier transformation (3 spatial domains, 1 time domain). The resulting 2H MR spectra were quantified with linear least-squares fitting of up to two Lorentzian lines and a linear baseline. Deuterium-enriched glycogen levels were overlaid with anatomical MR images as amplitude colormaps following spatial convolution with a Gaussian kernel.
Results
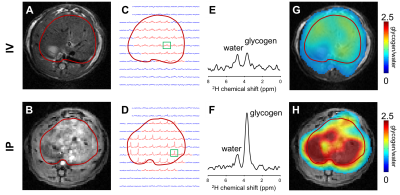
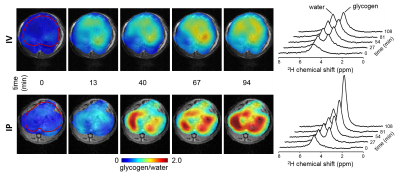
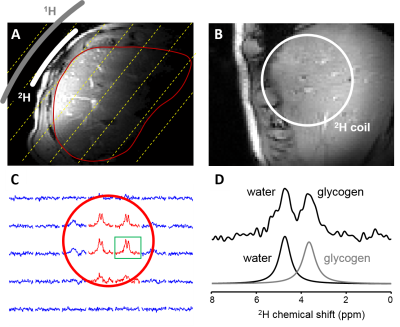
Figure 1 illustrates the acquisition of DMI data in rat liver during IV (top) and IP (bottom) infusion of [6,6’-2H2]-glucose, including MR images of liver, the 2H MRSI grid, individual 2H MR spectra and color-coded maps of labeled glycogen levels after 120 minutes of 2H-labeled glucose infusion. In Figure 2 the time course of glycogen synthesis is depicted, both as color coded map and as 2H MR spectra acquired without localization. The DMI data acquired at steady state from human liver are shown in Figure 3. The RF surface coil cannot provide complete liver coverage but allows the evaluation of the feasibility of DMI in human liver at 4T.Discussion
We show how 2H MRSI can be combined with administration of [6,6’-2H2]-glucose to measure glycogen synthesis in liver. Despite identical amounts of [6,6’-2H2]-glucose being provided, DMI of rat liver revealed that IP administration results in a higher rate of labeling and higher levels of labeled glycogen. This is in agreement with higher insulin levels in the portal vein compared to systemic circulation, and the concomitant stimulation of glycogen synthesis in liver (1,4). The rate of glycogen labeling during IP infusion of glucose appeared to be spatially inhomogeneous, with some areas of the liver showing initially higher levels of glycogen labeling than others, particularly during IP administration.
Data acquired at 4T show that DMI is also feasible at magnetic field strengths similar to those used for clinical MRI. 2H MR spectra at 4T show an anticipated reduced spectral resolution compared to 11.7T, but the spectral resolution remains sufficiently high to reliably separate water and glycogen signals.
Conclusion
DMI is a novel approach providing high spatial resolution data that can be used for metabolic studies in both animal models and human subjects. When using [6,6’-2H2]-glucose as a substrate DMI can noninvasively reveal differences in glycogen synthesis rates in liver.Acknowledgements
We thank Bei Wang and Xiaoxian Ma for assistance with animal preparation, and Terry Nixon and Scott McIntyre for maintenance and upgrades to the MR system.References
1. Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J. Clin. Invest. 2016;126:12–22. doi: 10.1172/JCI77812.
2. Sillerud LO, Shulman RG. Structure and metabolism of mammalian liver glycogen monitored by carbon-13 nuclear magnetic resonance. Biochemistry (Mosc.) 1983;22:1087–1094. doi: 10.1021/bi00274a015.
3. Petersen KF, Laurent D, Rothman DL, Cline GW, Shulman GI. Mechanism by which glucose and insulin inhibit net hepatic glycogenolysis in humans. J. Clin. Invest. 1998;101:1203–1209. doi: 10.1172/JCI579.
4. Samuel VT, Shulman GI. Nonalcoholic Fatty Liver Disease as a Nexus of Metabolic and Hepatic Diseases. Cell Metab. 2017;0. doi: 10.1016/j.cmet.2017.08.002.
Figures