0990
Hyperpolarized [1-13C]Pyruvate Magnetic Resonance Imaging of Placentae Associated With Intrauterine Growth Restriction1Medical Biophysics, Western University, London, ON, Canada, 2Robarts Research Institute, London, ON, Canada, 3Physiology and Pharmacology, Western University, London, ON, Canada, 4GE Healthcare, Toronto, ON, Canada, 5Obstetrics and Gynaecology, Western University, London, ON, Canada, 6Children's Health Research Institute, London, ON, Canada, 7Lawson Research Institute, London, ON, Canada
Synopsis
Intrauterine growth restriction (IUGR) is associated with impaired placental metabolism and transport. Hyperpolarized carbon-13 (HP13C) MRI was used to detect metabolic differences between control and IUGR placentae in pregnant guinea pigs, and to determine the impact of maternal hyperoxygenation on placental metabolism. Area under the curve (AUC) was calculated for lactate, alanine, and bicarbonate and expressed as a ratio relative to pyruvate AUC. The ratio of alanine to pyruvate AUC decreased significantly in IUGR versus control placentae. Maternal hyperoxygenation resulted in significant increases in ratios of alanine and bicarbonate to pyruvate AUCs for both control and IUGR placentae.
Introduction
Intrauterine growth restriction (IUGR) is associated with up to a 100-fold increase in perinatal mortality rates1. It is typically associated with placental insufficiency resulting in impaired placental gaseous exchange and nutrient transport. Hyperpolarized carbon-13 MRI (HP13C MRI) can be used to monitor metabolism in placentae2,3,4. Moreover, maternal hyperoxygenation has been used in IUGR to determine placental oxygenation response5. We propose that HP13C MRI can provide a means of assessing metabolic function in IUGR placentae and that IUGR placentae will respond differently than control placentae to maternal hyperoxygenation.Objectives
1) To detect metabolic differences between control and IUGR placentae in pregnant guinea pigs using hyperpolarized [1-13C]pyruvate MRI, and 2) to determine the impact of maternal hyperoxygenation on placental metabolism.Methods
Under a protocol approved by the institution’s Animal Care Committee, six pregnant guinea pigs underwent surgery at mid-gestation (31-33 days gestation, term ~69 days). Two sows (5 placentae) had vascular occluders placed on the uterine artery to restrict uteroplacental blood flow. The remaining four sows (12 placentae) underwent sham surgery.
MR imaging occurred at 53-64 days gestation (term 68 days) using a 3T MRI system (Discovery MR750, GE Healthcare). Animals were anaesthetized using isoflurane and air, a catheter was placed in the tarsal vein of the maternal hind paw. T1-weighted 1H images were obtained using a 32 coil cardiac array. HP13C imaging was performed with a custom-built 13C birdcage coil (Morris Instruments). [1-13C]pyruvic acid (Cambridge Isotope Laboratories) containing 15mM OX63 trityl radical (Oxford Instruments) and 1mM ProHance (Bracco) was hyperpolarized using a DNP polarizer (Hypersense, Oxford Instruments). 3.5mL of the hyperpolarized solution (80mM [1-13C]pyruvate, here on referred to as pyruvate) was injected over 12s. Time resolved 3D 13C images were acquired using a modified IDEAL pulse sequence6 (FOV=20cm, slice=8.5mm, matrix=24x24x14, BW=+/-8.9kHz, echoes=8, ETL=4, TE1=4.2ms, ΔTE=1.1ms). A double Gaussian radiofrequency pulse was used to excite pyruvate with a 1.5° flip angle, and the metabolic by-products with flip angles of 7.5°. Acquisition started 7.5s after the beginning of injection and images were acquired every 7.5s for a total of 45s. Maternal intake was changed from air to 100% oxygen (O2, hyperoxygenation) and 72+/-5min later the pyruvate injection and imaging were repeated. Sows were then recovered.
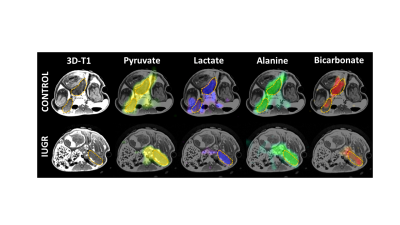
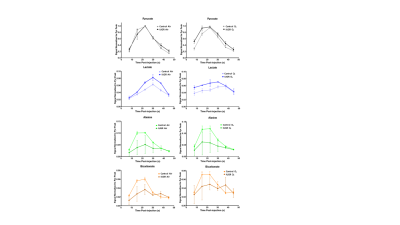
Fetal weight <80g (determined at collection at 65d gestation) was considered IUGR. HP13C images were normalized to peak pyruvate signal in each acquisition. Pyruvate, lactate, alanine, and bicarbonate 13C signal intensities were determined in ROIs encompassing total placental volumes using 3D Slicer (www.slicer.org). Area under the curve (AUC) was calculated for each metabolite and expressed as a ratio relative to pyruvate7. Unpaired t-tests were performed to determine significance for control versus IUGR and maternal air versus O2 for each metabolite using Prism (v7.03, GraphPad).
Results
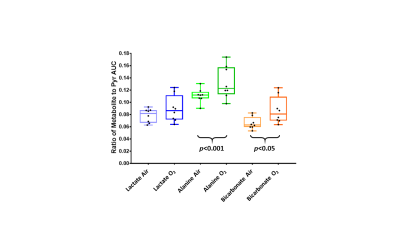
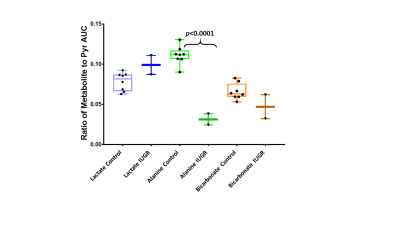
Eight fetuses in the sham surgery guinea pigs were >80g and two fetuses in the occluder surgery animals were <80g resulting in eight control and two IUGR placentae. Pyruvate, lactate, alanine, and bicarbonate were observed in all placentae (Figures 1 and 2). Significant differences in ratios of alanine to pyruvate (p<0.001) and bicarbonate to pyruvate (p<0.05) AUCs were observed between maternal air and hyperoxygenation conditions for both control and IUGR (Figure 3). A higher ratio of lactate to pyruvate AUCs, and lower ratios of bicarbonate and alanine to pyruvate AUCs were observed for IUGR compared to control placentae, but only the ratio of alanine to pyruvate AUC was significantly different (p<0.001) (Figure 2 and 4).Discussion
Previous reports have identified placental metabolism of pyruvate to lactate in HP13C MRI2,3,4. This study, to our knowledge, is the first to identify placental metabolism of pyruvate to lactate, alanine, and bicarbonate in HP13C images. Changes in ratios for lactate, alanine and bicarbonate to pyruvate AUCs with changes in maternal oxygenation indicate that it is possible to use HP13C MRI to measure a change in placental metabolism affected by maternal oxygen challenge. These changes were not significantly different for control compared to IUGR placentae; however, further experiments are needed to increase the number of IUGR placentae in this study. The reduction of alanine in the IUGR placenta suggests a movement of placental pyruvate into either acetyl-CoA and the TCA cycle or to lactate.Conclusions
HP13C MRI detected a significant decrease in ratio of alanine to pyruvate AUC in IUGR guinea pig placentae as compared to control. By exposing a pregnant guinea pig to hyperoxygenation, significant increases in ratios of alanine and bicarbonate to pyruvate AUCs were observed and this effect was not different for control compared to IUGR placentaeAcknowledgements
National Institutes of Health, U01 HD087181-01 and Canadian Institutes of Health Research, MOP-209113References
1. McPherson. Curr Opin Obstet Gynecol. 2013;25(2);152.
2. Friesen-Waldner et al. J Magn Reson Imaging. 2016;43(3):750-5.
3. Mikkelsen et al. R Soc Open Sci. 2017;4(4):161098.
4. Markovic et al. Proceedings of the Society of Magnetic Resonance in Medicine, 2017;3089.
5. Aimot-Macron Eur Radiol. 2013;23(5):1335-42.
6. Wiens et al. Magn Reson Med. 2015;74(6):1682-9.
7. Daniels et al. NMR in Biomedicine 2016;29.4:387-399.
Figures