0989
Quantitative geometric assessment of regional airway collapse in neonates via retrospectively respiratory-gated 1H UTE MRI1Center for Pulmonary Imaging Research, Cincinnati Children's Hospital, Cincinnati, OH, United States, 2Physics, Washington University in St. Louis, St. Louis, MO, United States, 3Upper Airway Center, Cincinnati Children's Hospital, Cincinnati, OH, United States, 4Pulmonary Medicine, Cincinnati Children's Hospital, Cincinnati, OH, United States, 5Radiology, Cincinnati Children's Hospital, Cincinnati, OH, United States, 6Medical Physics, University of Wisconsin - Madison, Madison, WI, United States, 7Radiology, University of Wisconsin - Madison, Madison, WI, United States, 8Neonatology and Pulmonary Biology, Cincinnati Children's Hospital, Cincinnati, OH, United States
Synopsis
Neonatal airway malacia (dynamic larynx, trachea, and/or bronchi collapse) is a common airway complication often associated with preterm birth and congenital abnormalities but has not been extensively studied. This condition is currently diagnosed through visual bronchoscopy, which can be unreliable and poses increased risks to patients. We address these issues with an innovative technique using retrospectively respiratory-gated ultrashort echo time MRI and geometric analysis of moving airway anatomy for regional, quantitative evaluation of dynamic airway collapse in quiet-breathing, non-sedated neonates. This method has the potential to yield more accurate and objective assessment of neonatal airway collapse than current techniques allow.
Purpose
Airway collapse (laryngomalacia [LM], tracheomalacia [TM], or bronchomalacia [BM]) is a common airway complication occurring in infants with bronchopulmonary dysplasia (BPD, lung disease of prematurity), congenital diaphragmatic hernia (CDH), or esophageal atresia/tracheoesophageal fistula (EA/TEF)1-6, but the underlying pathology, impact on clinical outcome, and response to therapy are not well understood. Infants with airway abnormalities typically face longer hospitalizations and increased medical comorbidities due to increased airway compliance during respiration5-7. The clinical diagnostic standard for collapse assessment is bronchoscopy, which has multiple potential limitations: only moderate inter-reader reliability; inconsistency between rigid and flexible scopes; lack of quantitative evaluation; and invasiveness, posing increased risk to patients. Computed tomography (CT) can be used to visualize collapse but has a time resolution limited by the gantry rotation and exposes infants to ionizing radiation. Further, both bronchoscopy and CT typically require sedation, which can obscure the airway dynamics under evaluation. In this study, we demonstrate the first dynamic 3D MR images of the neonatal airway and develop a novel method for non-invasive quantitative regional assessment of neonatal dynamic airway collapse under restful breathing conditions via retrospectively respiratory-gated ultrashort echo time (UTE) MRI, without sedation or ionizing radiation.Methods
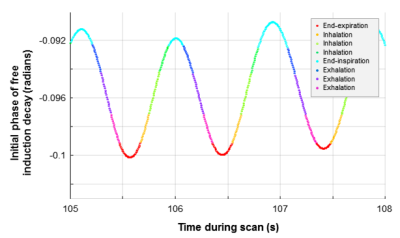
Neonatal 1H 3D radial UTE MR images8-9 were acquired during restful breathing in 14 neonatal subjects recruited from the NICU (40±2 weeks mean post-menstrual age) who also had clinical bronchoscopy (5 BPD, 4 CDH, 4 EA/TEF, 1 premature non-BPD with abnormal sleep study) using a quadrature body coil on a neonatal-sized, NICU-sited 1.5T scanner10-11, with no sedation administered for scanning. Expanding upon previous techniques12 using the time-course of k0 as a retrospective respiratory-gating waveform, UTE images were binned into eight frames throughout the respiratory cycle (Figure-1). Typical UTE parameters were: TR/TE≈5/0.2ms; FA=5º; FOV=18cm; number of radial projections=200,000; 3D isotropic resolution≈0.7 mm; and scan time≈16 min.
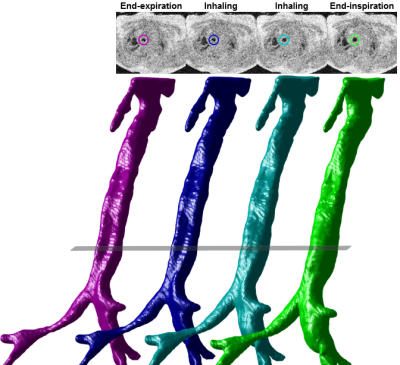
End-expiration airway anatomy was segmented from UTE images via ITK-SNAP (3.6.0, Penn Image Computing and Science Laboratory, USA)13. Airway motion during breathing was generated via image registration of respiratory-gated images14, yielding surface renderings throughout respiration (Figure-2). A center-line was defined through each respiratory-gated surface rendering via VMTK 1.2 (Orobix, Bergamo, Italy) to account for airway curvature and misalignment with axes15, and a series of disks bounded by the airway rendering and orthogonal to the center-line were defined at 1-mm intervals (Figure-3). The cross-sectional area (CSA) of each disk was calculated regionally along the airway, with degree of collapse defined here as:
$$Z_{collapse}=1-\frac{CSA_{end-expiration}}{CSA_{end-inspiration}}$$
such that Zcollapse=0 with no dynamic collapse and Zcollapse=1 with complete dynamic collapse, under conventional assumptions of intrathoracic collapse during expiration. We define presence of dynamic collapse when Zcollapsemax>0.25 at any point along the airway.
Results
Dynamic airway collapse was quantified
regionally in all cases, with collapse identified in 12 patients (Zcollapsemax>0.25) and absent in 2 patients (Zcollapsemax<0.25). Representative MRI results from two patients
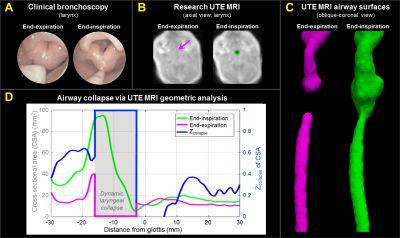
with collapse (both severe BPD) are presented, with qualitative comparison to
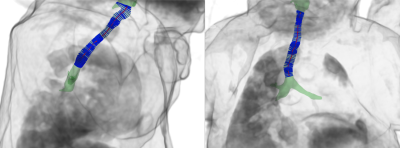
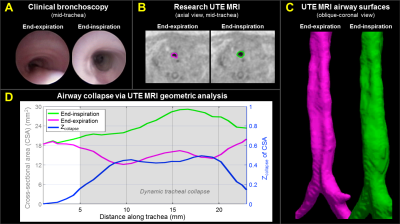
clinical bronchoscopy. Subject A (Figure-4) demonstrated partial dynamic collapse in the middle and lower trachea
(TM) that progressively worsened during exhalation and reached maximum collapse
at end-expiration (maximum Zcollapse≈0.5). Subject B (Figure-5) demonstrated little collapse through the trachea (Zcollapse≈0-0.4), partial collapse above the larynx (Zcollapse≈0-0.4), and complete airway collapse (Zcollapse=1) in the larynx (LM),
occurring dynamically at end-expiration only. These results regionally agree
with qualitative findings from bronchoscopy.
Discussion and Conclusions
This innovative MRI technique provides a new platform for quantitative and regional assessment of dynamic airway morbidities in neonates during restful breathing, without requiring sedation, invasive procedures, or ionizing radiation, and qualitatively compares well to clinical bronchoscopy. While rigorous validation with bronchoscopic findings is required to establish accuracy, this method overcomes the current barriers associated with existing diagnostic standards, yielding more objective and quantitative neonatal airway biomarkers than currently possible. Further, this technique poses no additional risks to patients and so can be used in serial assessment to guide use of pharmacologic interventions for airway collapse, such as albuterol. In the future, neonatal dynamic airway imaging may be used in conjunction with computational fluid dynamics (CFD) models14 to calculate respiratory effort and predict increased energy expenditure in infants related to airway collapse.
Looking forward, regional and dynamic biomarkers from geometric analysis and CFD simulations may be associated with clinical outcomes, which would allow for direct translation to the clinical care of neonatal patients with airway complications. This may have particular impact with BPD, for which the connection between pulmonary and airway disease is not yet well characterized. Ultimately, this approach has the potential to significantly advance understanding and treatment of airway morbidities and trajectories in this vulnerable neonatal population.
Acknowledgements
The authors thank The Perinatal Institute at Cincinnati Children’s Hospital Medical Center, The Hartwell Foundation, NIH P01 HD093363, and The Departments of Radiology and Medical Physics and the School of Medicine and Public Health at University of Wisconson-Madison for research funding and support.References
- Downing GJ, Kilbride HW. Evaluation of airway complications in high-risk preterm infants: application of flexible fiberoptic airway endoscopy. Pediatrics. 1995;95(4):567-72.
- Miller RW, Woo P, Kellman RK, Slagle TS. Tracheobronchial abnormalities in infants with bronchopulmonary dysplasia. The Journal of pediatrics. 1987;111(5):779-82.
- Cohn RC, Kercsmar C, Dearborn D. Safety and efficacy of flexible endoscopy in children with bronchopulmonary dysplasia. American journal of diseases of children. 1988;142(11):1225-8.
- McCubbin M, Frey EE, Wagener JS, Tribby R, Smith WL. Large airway collapse in bronchopulmonary dysplasia. The Journal of pediatrics. 1989;114(2):304-7.
- Hysinger EB, Friedman NL, Padula MA, Shinohara RT, Zhang H, Panitch HB, Kawut SM, Children's Hospitals Neonatal Consortium. Tracheobronchomalacia Is Associated with Increased Morbidity in Bronchopulmonary Dysplasia. Ann Am Thorac Soc. 2017 Jun 16. doi: 10.1513/AnnalsATS.201702-178OC.
- Hysinger EB, Panitch HB. Paediatric Tracheomalacia. Paediatr Respir Rev. 2016;17:9-15. Baxter JD, Dunbar JS. Tracheomalacia. The Annals of otology, rhinology, and laryngology. 1963;72:1013-23.
- Baxter JD, Dunbar JS. Tracheomalacia. The Annals of otology, rhinology, and laryngology. 1963;72:1013-23.
- Hahn AD, Higano NS, Walkup LL, Thomen RP, Cao X, Merhar SL, Tkach JA, Woods JC, Fain SB. Pulmonary MRI of neonates in the intensive care unit using 3D ultrashort echo time and a small footprint MRI system. J Magn Reson Imaging. 2017;45(2):463-71.
- Higano NS, Fleck RJ, Spielberg DR, Walkup LL, Hahn AD, Thomen RP, Merhar SL, Kingma PS, Tkach JA, Fain SB, Woods JC. Quantification of neonatal lung parenchymal density via ultrashort echo time MRI with comparison to CT. J Magn Reson Imaging. 2017 Oct;46(4):992-1000.
- Tkach JA, Hillman NH, Jobe AH, Loew W, Pratt RG, Daniels BR, Kallapur SG, Kline-Fath BM, Merhar SL, Giaquinto RO, Winter PM, Li Y, Ikegami M, Whitsett JA, Dumoulin CL. An MRI system for imaging neonates in the NICU: initial feasibility study. Pediatr Radiol. 2012;42(11):1347-56.
- Tkach JA, Merhar SL, Kline-Fath BM, Pratt RG, Loew WM, Daniels BR, Giaquinto RO, Rattan MS, Jones BV, Taylor MD, Tiefermann JM, Tully LM, Murphy EC, Wolf-Severs RN, LaRuffa AA, Dumoulin CL. MRI in the neonatal ICU: initial experience using a small-footprint 1.5-T system. AJR Am J Roentgenol. 2014;202(1):W95-W105.
- Higano NS, Hahn AD, Tkach JA, Cao X, Walkup LL, Thomen RP, Merhar SL, Kingma PS, Fain SB, Woods JC. Retrospective respiratory self-gating and removal of bulk motion in pulmonary UTE MRI of neonates and adults. Magn Reson Med. 2017;77(3):1284-95.
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006 1;31(3):1116-28.
- Bates AJ, Schuh A, Amine-Edine G, McConnell K, Loew W, Fleck RJ, Woods JC, Dumoulin CL, Amin RS. Assessing the Relationship Between Movement and Airflow in the Upper Airway using Computational Fluid Dynamics with Motion Determined from Magnetic Resonance Imaging. Clinical Biomechanics 2017. PII: S0268-0033(17)30223-1. DOI: doi:10.1016/j.clinbiomech.2017.10.011.
- Piccinelli M, Veneziani A, Steinman DA, Remuzzi A, Antiga L. A framework for geometric analysis of vascular structures: application to cerebral aneurysms. IEEE Trans Med Imaging. 2009;28(8):1141-55.
Figures