0986
Effect of motor planning and dopaminergic medication on cerebellar network connectivity during dual motor tasking in Parkinson's disease1HMCS, NINDS - NIH, Bethesda, MD, United States, 2Neurologie, CHUV, Lausanne, Switzerland, 3Department of Psychiatry, University of Cambridge, Cambridge, United Kingdom, 4ICM-CRICM, UPMC/INSERM, UMR_975, CNRS 7225, Paris, France
Synopsis
We investigated cerebellar deficits in dual-motor-tasking in Parkinson’s disease (PD) patients. Eighteen PD patients (scanned ON and OFF dopaminergic medication) and 18 matched controls performed simultaneous finger movements in a coupled or individuated fashion, and with different visual cues at 3T. We showed that cerebello-striatal network interactions play a role in symptomatic dual tasks in PD, and is influenced by dopaminergic medication. Our data suggest that cerebellar-striatal loop is involved in planning fine dexterous tasks without interacting with the cortical motor areas.
INTRODUCTION
Parkinson’s disease (PD) patients experience problems with dual tasking, whether involving simultaneous cognitive and motor (talking while walking), or two motor tasks. These impairments are thought to be associated with difficulties in motor planning and slowness of motor execution,1 and attributed to basal ganglia dysfunction2 or abnormal involvement of the cerebellum.3 While, dual task performance does not improve with dopaminergic medication,4 sensory cueing helps PD patients to overcome their motor planning difficulties.5,6 Here, we investigated the intereaction between the cerebellum and basal ganglia in dual tasking.METHODS
Subjects (18PD/18HV) performed visuo-motor control tasks using the right index (rIF) and middle (rMF) fingers to control two vertical cursors on a screen via an MRI-compatible force-sensitive device. The task was “individuated” when rIF followed a trace while rMF kept a constant value, or “coupled”, when the fingers followed the same trace. Motor planning was manipulated by the visual cueing: the trace scrolled from right to left on the screen for 3.5 s (V+) or 150 ms (V-) before it reached the cursor (Fig.1). Tasks were presented in blocks, with passive viewing as control. After training, subjects underwent four 11min10s fMRI runs in a 3T GE scanner. The four conditions were presented in randomized order. Patients had two sessions separated by 24 hours in a randomized order: on dopaminergic medication (ON) and after 12h medication withdrawal (OFF). MPRAGE was collected for registration. Data were processed using SPM8. Statistics included: (i) 2x2x2 ANOVA (Group: HV, PD OFF; Task: Coupled, Individuated; Cueing: V+,V-); (ii) 2x2x2 ANOVA (Medication: ON, OFF; Task: Coupled, Individuated; Cueing: V+,V-), and psychophysiologic interactions (PPI) model.7,8 Accuracy of motor output and degree of finger coupling were used as covariates. Results were FDR-corrected at p<0.05.RESULTS
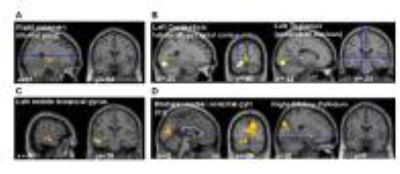
Patients and HVs performed the task with similar accuracy (p=0.84). However, the finger coordination differed for the dual tasks with PD having worse performance than HV, especially for the Coupled task (F=8.65, p=0.04). L-DOPA improved finger coordination for the Individuated task (F=14.2, p=0.01). Visual cueing did not influence motor performance (p=0.09). The brain activation showed significant main effects but no interaction. The right cerebellar lobule VI was less active in PD compared to HV (main effect of Group, Fig.2A, yellow); more active during the Coupled compared to Individuated task (main effect of Task, Fig.2A, red); and more active during ON compared to OFF (main effect of Medication, Fig.2B). Patients ON medication had also greater activation in the bilateral occipital cortex and the right globus pallidum. The right cerebellar lobule VI was defined as volume of interest, seeding the functional connectivity analysis. This showed a main effect of Group, and interactions Group x Cueing and Medication x Cueing (Fig.3A-D). Main effect of Group: PD, compared to HV, had decreased connectivity in a network involving the right intraparietal sulcus and the right putamen (Fig.3A). Main effect of Cueing: V+ compared to V- showed an increase in connectivity in a network involving the left medial primary visual cortex. Interaction Group x Cueing: decreased connectivity in PD during V+ compared to V-, while the opposite was observed in HV, in a network involving the bilateral associative visual cortex and the left geniculate nucleus (Fig.3B). In PD, dopaminergic medication affected cerebellar connectivity, showing the following effects: Main effect of Medication: decreased connectivity in ON compared to OFF in a network involving temporal and parietal areas (Fig.3C); interaction Medication x Cueing: patients showed increased connecitivty during V+ versus V- during ON (while the opposite was observed during OFF) in a network involving the left visual cortex and the right globus pallidum (Fig.3D).DISCUSSION
Difficulties to perform dual motor tasks involving visuomotor integration are associated with cerebellar dysfunction, and abnormal connectivity with the thalamo-occipital network. Dopaminergic treatment improves striatal involvement and increases its communication with the cerebellum and visual cortical areas, without affecting cortical motor areas. Recent studies showed that the cerebellum can influence dopaminergic striatal activity.9 Our study shows that, in the presence of dopamine, the cerebellum can improve pallidal involvement during symptomatic dual tasks.CONCLUSION
We showed that cerebello-striatal network interactions play a role in symptomatic dual tasks in PD. Our data suggest that altered communication between the cerebellum and the striatum in PD has an effect on cerebellar-striatal loop without involving cortical motor areas.Acknowledgements
This work was supported by the NINDS Intramural Research Program.References
1. Wu T, Hallett M. The cerebellum in Parkinson's disease. Brain. 2013;136(Pt 3):696-709
2. Nieuwhof F, Bloem BR, Reelick MF, Aarts E, Maidan I, Mirelman A, et al. Impaired dual tasking in Parkinson's disease is associated with reduced focusing of cortico-striatal activity. Brain. 2017;140(5):1384-98
3. Gao L, Zhang J, Hou Y, Hallett M, Chan P, Wu T. The cerebellum in dual-task performance in Parkinson's disease. Sci Rep. 2017;7:45662
4. Azulay JP, Mesure S, Blin O. Influence of visual cues on gait in Parkinson's disease: contribution to attention or sensory dependence? J Neurol Sci. 2006;248(1-2):192-5.
5. Schubert M, Prokop T, Brocke F, Berger W. Visual kinesthesia and locomotion in Parkinson's disease. Mov Disord. 2005;20(2):141-50.
6. Pieruccini-Faria F, Jones JA, Almeida QJ. Insight into dopamine-dependent planning deficits in Parkinson's disease: A sharing of cognitive & sensory resources. Neuroscience. 2016;318:219-29.
7. Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218-29
8. Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19(1):200-7.
9.
Delis DC, Jacobson M, Bondi MW, Hamilton JM,
Salmon DP. The myth of testing construct validity using factor analysis or
correlations with normal or mixed clinical populations: lessons from memory
assessment. J Int Neuropsychol Soc. 2003;9(6):936-46.
Figures
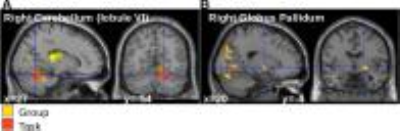
Figure 2: Activation analysis. A. Main effect of Group (yellow, Z score=4.69) and main effect of Task (red , Z score=5.26) in the
right cerebellum (p<0.05 FDR correction over the whole brain). B. Main
effect of medication in the right globus pallidum (ON>OFF, Z score = 3.94,
p<0.001 uncorrected at the level of the whole brain; p<0.05 with FDR
correction at the cluster level).