0985
Altered brain network structure during urethane-induced sleep states in a rat model of early-stage Parkinson’s disease1A.I.Virtanen Institute, University of Eastern Finland, Kuopio, Finland, 2School of Pharmacy, University of Eastern Finland, Kuopio, Finland, 3Moscow Institute of Physics and Technology, Moscow, Russian Federation, 4Institute of Theoretical and Experimental Biophysics, Pushchino, Russian Federation
Synopsis
6-hydroxydopamine (6-OHDA) striatal lesion is a well-established rat model of early-stage Parkinson’s disease. The aim of the study was to compare connectivity patterns between 20 6-OHDA lesion rats and 10 sham controls under urethane anesthesia, modelling natural sleep, by using functional magnetic resonance imaging (fMRI). We found that functional connectivity patterns were disturbed in lesion animals. The decrease in functional connectivity, however, occurred only in rapid eye movement (REM)-like state. Furthermore, thalamocortical functional connectivity was correlating with striatal dopamine depletion ratio, making these changes possible early diagnostic markers for Parkinson’s disease.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by degeneration of dopaminergic neurons in the substantia nigra pars compacta leading to a variety of motor and non-motor symptoms. One class of the non-motor symptoms is sleep disturbances, typically appearing several years before the onset of motor deficits1. Currently, the most common approach to model early-stage PD in rodents is the unilateral intracerebral injection of 6-hydroxydopamine (6-OHDA) into the striatum2. It initiates a process of neuronal degeneration in nigrostriatal pathway, which leads to dopamine depletion in striatum mimicking the pathophysiology of PD. The effects of the partial lesioning on sleep are not well characterized. Resting-state functional magnetic resonance imaging (rsfMRI) is a powerful tool to explore whole-brain large-scale connectivity3. However, studying natural sleep in animal models in magnet is practically impossible due to the stress of animals induced by restrainment, sounds, and vibration. Recent studies have exploited sleep models, such as urethane anesthesia4,5. As the combination of partial striatal 6-OHDA lesion rat model and urethane-induced sleep could provide a relevant platform for preclinical studies and drug development for the early-stage PD, the aim of this study was to investigate whether any changes in functional connectivity (FC) during sleep, similar to early-stage PD patients, could be observed in preclinical experimental setup.Materials and methods
The study was conducted in 20 lesioned and 10 sham-operated male Wistar rats weighing 270 – 360 g. Animals underwent partial unilateral striatal lesion as described previously6. Five weeks after the 6-OHDA or sham lesion, each animal was imaged with spin-echo echo-planar imaging sequence (TR = 2 s, TE = 45 ms, resolution 0.5x0.5x0.9 mm, 17 slices) for 40 minutes under urethane anesthesia (1.25 g/kg i.p.). Breathing, heart rate and temperature were monitored. After rsfMRI scanning, the animals were decapitated, both striata were dissected for dopamine level analysis by a high performance liquid chromatography with electrochemical detection as previously described6. Sleep-like states were detected using breathing rate fluctuations, as described by Wilson et al4. Functional connectivity patterns were compared between lesion and sham animals in NREM-like and REM-like states using set of 17 regions of interest. FC was calculated based on Pearson’s correlation coefficient and compared between groups after Fisher’s z-transform using t-test with false discovery rate correction for multiple comparisons. Additionally, correlation between thalamocortical connectivity and dopamine levels were investigated.Results
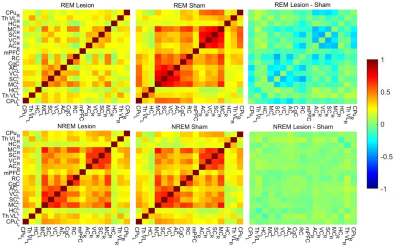
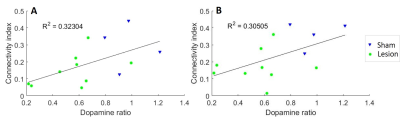
In 4/10 sham animals and in 9/20 lesion animals we observed clear state changes between REM-like and NREM-like state. Other animals were treated as being in NREM-like state7. We found that FC patterns were disturbed in 6-OHDA lesioned animals compared to sham rats. Interestingly, these disturbances depended on the sleep-like state (Fig. 1). During REM-like state, corticocortical and corticostriatal connections were substantially decreased in 6-OHDA lesion animals (Fig. 2). In contrast, during NREM-like state, differences in FC between sham and lesion animals were not significant (p > 0.8). In addition, the strength of ipsilateral thalamocortical connectivity correlated with dopamine depletion ratio (Fig. 3) during REM-like state (p < 0.05), but not during NREM-like state (p > 0.45).Discussion
We found that brain FC patterns under urethane anesthesia differed between striatal 6-OHDA and sham rats during the REM-like state. The observed changes had similarities with clinical findings in PD patients measured during wakefulness: decreased FC from putamen to sensorimotor cortex and contralateral putamen is observed already in early stage of the disease8. In patients with average and advanced stage of the disease decreased corticocortical and corticostriatal connectivity is observed9,10. The possible reason for connectivity patterns being different only in one sleep-like state is the direct participation of DA to sleep regulation. In support of that, the strength of thalamocortical connectivity during REM-like state correlated with the dopamine levels in the striatum. Further, the correlation between dopamine ratio and thalamocortical connectivity implies that assessment of these connections may serve as indirect biomarkers for the extent of dopamine depletion in the striatum.Conclusion
This study shows that the unilateral striatal 6-OHDA lesion rat model may serve as a preclinical model for sleep disturbances observed in early-stage PD. More importantly, it also holds promise as a model for developing and testing the effects of novel neurorestorative treatments for early-stage PD.Acknowledgements
This work was supported by the Finnish Academy of Science and Letters.References
1. Bargiotas P, Schuepbach MWM, Bassetti CL. Sleep-wake disturbances in the premotor and early stage of Parkinsonʼs disease. Curr Opin Neurol. 2016;29: 763–772.
2. Simola N, Morelli M, Carta AR. The 6-hydroxydopamine model of Parkinson’s disease. Neurotox Res. 2007; 11: 151–167.
3. Biswal B, Yetkin FZ, Haughton VM et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995, 34(4): 537–541.
4. Wilson DA, Hoptman MJ, Gerum S V et al. State-dependent functional connectivity of rat olfactory system assessed by fMRI. Neurosci Lett. 2011, 497: 69–73.
5. Zhurakovskaya E, Paasonen J, Shatillo A et al. Global Functional Connectivity Differences between Sleep-Like States in Urethane Anesthetized Rats Measured by fMRI. PLOS ONE. 2016; 1–12.
6. Leikas J V., Kääriäinen TM, Jalkanen AJ et al. Combined ipsilateral limb use score as an index of motor deficits and neurorestoration in parkinsonian rats. J Neurosci Res. 2017. 00: 1–13.
7. Gretenkord S, Rees A, Whittington MA et al. Dorsal versus ventral differences in fast Up-state associated oscillations in the medial prefrontal cortex (mPFC) of the urethane anaesthetised rat. J Neurophysiol. 2016; jn.00762.2016.
8. Luo CY, Song W, Chen Q et al. Reduced functional connectivity in early-stage drug-naive Parkinson’s disease: A resting-state fMRI study. Neurobiol Aging. 2014; 35(2):431–41.
9. Sharman M, Valabregue R, Perlbarg V et al. Parkinson’s disease patients show reduced cortical-subcortical sensorimotor connectivity. Mov Disord. 2013;28(4):447–54.
10. Hacker CD, Perlmutter JS, Criswell SR et al. Resting state functional connectivity of the striatum in Parkinson’s disease. Brain. 2012;135(12):3699–711.
Figures