0982
Environmental Paraquat and HFE genetics as factors in the development of Parkinson’s disease1Neurosurgery, The Pennsylvania State University - College of Medicine, Hershey, PA, United States, 2Radiology, The Pennsylvania State University - College of Medicine, Hershey, PA, United States
Synopsis
The herbicide paraquat (PQ) is believed to have a neurotoxic role in the development of Parkinson’s disease (PD) as there is increased risk of exposure in rural areas. The HFEH63D polymorphism has been shown to cause brain abnormalities and disrupt iron homeostasis, key components of PD etiology. This work hypothesized that HFEH67D carrier status would augment PQ induced PD histological and imaging metrics. The R2 and histology data from PQ treated mice provide evidence that PQ had a marked effect in the WT but not HFEH67D mice, suggesting that the H67D polymorphism imparts a neuroprotective role in PD.
Introduction
The herbicide paraquat (PQ) is believed to have a neurotoxic effect in the development of Parkinson’s disease (PD) due to its ability to increase iron uptake, produce superoxide radicals, and initiate dopaminergic cell death in the substantia nigra.1-4 The HFEH67D mouse polymorphism, analogous to the human HFEH63D polymorphism, has been shown to disrupt iron homeostasis and cause brain abnormalities in both H67D mouse and H63D human carriers.5,6 The H63D and H67D polymorphisms are known to disrupt iron homeostasis and cause brain abnormalities due to an increase in oxidative stress within the brain. 5-7. Many PD studies have focused on the dopaminergic nigro-striatal pathway and its associated structures, due to its degeneration in PD. However, recent studies have mapped connections from the subthalamic nucleus, zona incerta, and other hypothalamic areas to dopaminergic neurons within the substantia nigra pars compacta (SNc) and their relation to PD pathogenesis.8 This work hypothesized that HFEH67D carrier status would augment PQ-induced histological and imaging metrics known to be associated with PD.Methods
31 four-month-old mice were used in this study design: 16 HFEWT and 15 homozygous HFEH67D knock-ins. Mice were housed under a standard light cycle, fed ad libitum, and cared for in accordance with IACUC policies. In this four-week study, mice were injected IP weekly with PQ (10 mg/kg) (N=16) or saline (N=15) for three weeks. MRI relaxation (R2) and diffusions maps were obtained at baseline and study end. Imaging was performed using a Bruker 7T system and parametric maps were computed using in-house software. Immunohistological staining for tyrosine hydroxylase (TH), cleaved caspase-3, L-ferritin, and iron positive cells as well as astrocyte (GFAP) and microglia proliferation (IBA1) were performed on harvested tissue.Results
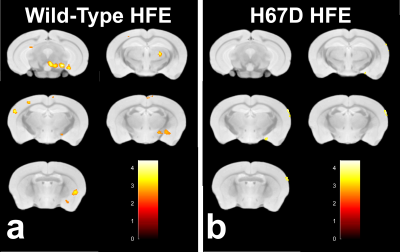
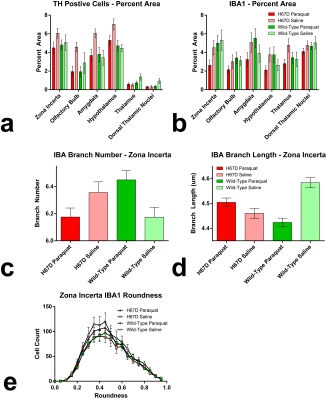
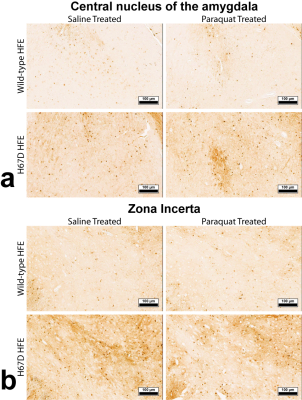
Relaxation maps showed an R2 increase in the WT mice given PQ injections within the SNc, ventral tegmental area as well as thalamic, amygdalar, and hypothalamic nuclei compared to WT animals given saline. This increase in R2 was not seen between the H67D paraquat and saline groups (Fig. 1). The WT mice performed more poorly on the behavioral tests after being treated with PQ, whereas no behavioral differences were seen in the HFEH67D mice. IBA1 staining showed a consistent decrease in the amount of microglia cells in the H67D PQ mice compared to the other treatment groups. TH was most abundant in the H67D saline mice within the zona incerta, subthalamic nucleus, olfactory, and amygdala areas (Fig. 2). These same regions showed a consistent decrease in TH in the H67D PQ animals. However, TH-containing neurons were sparse within the thalamus and dorso-thalamic nuclei in the H67D PQ, H67D saline, and WT PQ animals when compared to the WT saline group. The Perl’s stain for iron revealed that iron was more heavily deposited in the H67D treatment groups within the central amygdala and zona incerta (Fig. 3).Discussion
The increase in R2 values within the WT treatment groups is believed to be due to an increase in inflammation, loss of cellularity, and alteration in iron homeostasis. The lack of increased R2 in the H67D treatment groups led us to hypothesize that the H67D polymorphism provided a neuroprotective effect to those animals. Therefore, IHC was used to look for cellular markers of inflammation and loss of cellular structure. IBA1 staining showed high levels of microglia in the WT PQ group versus the WT saline group, indicating that the PQ injections caused inflammation and loss of cellular structure within the hypothalamus, amygdala, and olfactory regions. However, within the H67D animals, the PQ group had fewer microglia cells than the saline group. This could be due to differentiation of neural stem cells into astrocytes, oligodendrocytes, or microglia9; a process which is altered in H67D precursor cells. The H67D PQ animals had less TH-positive neurons than the H67D saline group, but were however, similar to the WT PQ and saline quantifications in all regions except for the zona incerta and subthalamic nucleus. This suggests that even though the IBA1 staining shows less potential signs of inflammation in the H67D PQ animals, dopamine neurons were still affected by the PQ injections.Conclusion
The R2 and histology data provide evidence that paraquat had a marked effect on the WT, but not the H67D knock-in mice. The decrease in TH-positive neurons paired with the apparent increase in iron-containing cells in the H67D animals suggests that although that the H67D polymorphism provides a neuroprotective role in a PQ-induced PD model, other neurons are still effected by the herbicide PQ.Acknowledgements
No acknowledgement found.References
1. Cocheme HM, Murphy MP. Complex I is the major site of mitochondrial superoxide production by paraquat. J Biol Chem. Jan 25 2008;283(4):1786-1798.
2. Wang A, Costello S, Cockburn M, Zhang X, Bronstein J, Ritz B. Parkinson's disease risk from ambient exposure to pesticides. Eur J Epidemiol. Jul 2011;26(7):547-555.
3. Thiruchelvam M, Brockel BJ, Richfield EK, Baggs RB, Cory-Slechta DA. Potentiated and preferential effects of combined paraquat and maneb on nigrostriatal dopamine systems: environmental risk factors for Parkinson's disease? Brain Res. Aug 11 2000;873(2):225-234.
4. Yin L, Lu L, Prasad K, et al. Genetic-based, differential susceptibility to paraquat neurotoxicity in mice. Neurotoxicol Teratol. May-Jun 2011;33(3):415-421.
5. Meadowcroft MD, Wang J, Purnell CJ, et al. Reduced White Matter Integrity in Cognitively Normal HFE Mutation Carriers. Proc. Intl. Soc. Mag. Reson. Med. 2016;24:3413.
6. Meadowcroft MD, Wang J, Purnell CJ, et al. Reduced Cerebral White Matter Integrity Assessed by DTI in Cognitively Normal H63D-HFE Polymorphism Carriers. J Neuroimaging. Aug 03 2017.
7. Ali-Rahmani F, Schengrund CL, Connor JR. HFE gene variants, iron, and lipids: a novel connection in Alzheimer's disease. Front Pharmacol. 2014;5:165.
8. Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. Jun 07 2012;74(5):858-873.
9. Sher F, Rossler R, Brouwer N, Balasubramaniyan V, Boddeke E, Copray S. Differentiation of neural stem cells into oligodendrocytes: involvement of the polycomb group protein Ezh2. Stem Cells. Nov 2008;26(11):2875-2883.
Figures