0981
Detection of Medication-Induced Changes in Thalamic GABA in Patients with Parkinson’s Disease Using J-Edited Spectroscopy1Neurology, Vanderbilt University Medical Center, Nashville, TN, United States, 2Biostatistics, Vanderbilt University, Nashville, TN, United States, 3Radiology, Vanderbilt University Medical Center, Nashville, TN, United States
Synopsis
A major neurochemical regulator of the thalamocortical network is GABA. We used J-edited MRS with compartment correction to evaluate the pharmacological effect of Dopamine agonist (DAA) therapy on thalamic GABA in patients with Parkinson’s disease (PD). Our findings suggest that DAA alters GABA release in PD patients, and that medication-induced changes may be associated to the presence of impulsive behaviors. These results provide evidence that J-edited MRS can be used to measure subcortical neurotransmitter concentration non-invasively, allowing the investigation of the pharmacological effects in GABAergic activity in humans.
Introduction
Dopamine agonist (DAA) medications target D2-like receptors, which subserve the thalamocortical neural network. One major neurochemical modulator of this network is GABA. Edited Magnetic Resonance Spectroscopy (MRS) offers the unique possibility to investigate the pharmacological effects of DAA in humans by providing a non-invasive measurement of changes to GABA concentration. In this study, we used J-edited MRS with partial volume correction applied to the subcortex to evaluate the effect of DAAs on thalamic GABA in patients with Parkinson’s disease (PD). We hypothesize that a dysfunctional GABAergic response to DAA therapy may be associated with the manifestation of impulsive and compulsive behaviors (ICBs) in PD patients.Methods
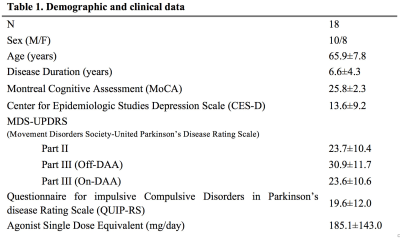
Participants: We examined 18 patients with PD that were taking DAA medication. A neurologic exam was performed on all participants, and they all completed the Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease-Rating Scale (QUIP-RS), a rating scale designed to measure severity of ICBs symptoms. Demographic and clinical data are shown in Table 1.
Image Acquisition: Patients were scanned in the Off- and On-DAA state using a 3T MRI scanner (Achieva, Philips Healthcare, the Netherlands) with body coil transmit and 32-channel head coil reception. Scanning included a 3D structural T1-weighted whole brain image, (MPRAGE, TR/TE=8.9/4.6 ms; turbo gradient echo factor=131; spatial resolution=1x1x1 mm3), and single-voxel J-edited MRS using MEGA-PRESS (TR/TE=3000/68 ms; 320 transients; 2048 data points at a spectra width of 2 kHz; voxel dimensions=30x22x28 mm3). The spectroscopy voxel was placed in the right thalamic area. Editing pulses (14 ms, 140 Hz bandwidth) were applied at 1.9 ppm and 8 ppm on alternate scans. An unedited MRS scan without water suppression was also acquired for normalization.
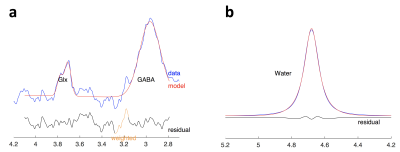
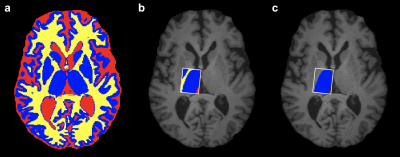
Image Analysis: MRS analysis was performed using Gannet 3.0. 1 Frequency and phase correction and outlier rejection was applied. The corrected spectrum was processed to obtain the area under the edited GABA+ (i.e. GABA + macromolecules) signal at 3 ppm (Figure 1a), and the unsuppressed water spectra was processed to obtain the area under the water peak (Figure 1b), which were then used to estimate GABA+ concentration relative to water. To account for the underlying tissue composition, we applied the a-correction 2,3; GannetCoRegister was used to register the MRS voxel to the T1-weighted image, and tissue segmentation was performed by merging the results obtained from FSL FAST and FSL FIRST (Figure 2a). The MRS voxel mask was then applied to the tissue segmentation to determine the tissue voxel fractions for gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) (Figure 2b). The compartment correction (using a=0.5, Wanasapura values for relaxation parameters, and 36.1 mol/dm3, 43.3 mol/dm3, and 53.8 mol/ dm3 for MR-visible water concentrations for WM, GM, and CSF, respectively), and tissue normalization (equation (5) from 2) were then applied to account for differences in GABA+/H2O concentration between GM and WM. Finally, the MRS voxel fraction corresponding to the thalamus (fthal) was calculated using the right thalamic mask from FSL FIRST (Figure 2c), and it was preserved for statistical analysis.
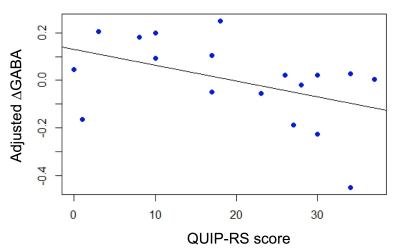
Statistical Analysis: The effect of DAAs on thalamic GABA was estimated as ΔGABA=(GABA+ON – GABA+OFF)/GABA+OFF, where GABA+ON,OFF represent the GABA+ estimate in the On- and Off-DAA conditions. To understand whether GABA responses were related to a quantitative marker of impulsivity, we performed a regression analysis specifying ΔGABA as dependent variable, QUIP-RS score as independent variable, and age and fthal as covariates.
Results
Voxel tissue composition was different across subjects, with voxel fractions varying between 0.29-0.48, 0.48-0.61, and 0.03-0.17 for WM, GM and CSF, respectively. Correcting for voxel composition and compartment-specific water relaxation resulted in significantly increased GABA+ values (t-tests P<0.0001), but the corrected and uncorrected values were highly correlated (R2=0.98). The regression analysis showed that, in our cohort of PD patients, greater impulsivity, as indicated by higher QUIP-RS score, was associated with lower ΔGABA (Figure 3).Discussion
We used MRS to measure GABA+ concentration in the thalamus to quantitatively evaluate the GABAergic response to DAA medication in a cohort of PD patients. Our findings show that we can assess medication induced changes to thalamic GABA, and these changes differ in patients with more severe impulsive behaviors. These results also provide evidence that J-edited MRS with tissue correction can be used to measure subcortical neurotransmitter concentration non-invasively, allowing the investigation of the pharmacological effects in GABAergic activity in humans and its association with the clinical manifestation of neurological diseases.Acknowledgements
This study was supported by the NIH/NINDS R01 NS097783 and K23 NS080988.
We thank all the volunteers who participated in this study, Grace Tipps for her support in patient recruitment, and Kristen George-Durrett, Leslie McIntosh, Clair Jones, and Christopher Thompson for their assistance with the data acquisition.
References
1. Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging. 2014;40:1445–1452.
2. Harris AD, Puts NAJ, Edden RAE. Tissue correction for GABA-edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. J Magn Reson Imaging. 2015;42:1431–1440.
3. Porges EC, Woods AJ, Lamb DG, et al. Impact of tissue correction strategy on GABA-edited MRS findings. Neuroimage. 2017;162:249-256.
Figures