0972
Dependence of the Chemical Shift of 129Xe Dissolved in Red Blood Cells on Transit Time from the Lung Gas Exchange Region in Rats1Translational Medicine, Hospital for Sick Children, Toronto, ON, Canada, 2Medical Biophysics, University of Toronto, Toronto, ON, Canada, 3Medical Imaging, University of Toronto, Toronto, ON, Canada
Synopsis
The chemical shift of 129Xe dissolved in red blood cells was measured at increasing transit times from the gas exchange region of the lungs of healthy rats and rats exposed to hyperoxia/hypoxia, a model of bronchopulmonary dysplasia. The results show that the chemical shift decreased with increasing transit times. Furthermore, the chemical shift was different between the healthy and exposed cohorts. A possible mechanism for this phenomenon based on changes in bulk magnetic susceptibility distal to the lungs is proposed.
Introduction
It has been demonstrated that the chemical shift (CS) of 129Xe dissolved in red blood cells (RBCs) is dependent on transit time from the gas exchange region of the lungs in healthy humans1. In this study we demonstrate this effect in healthy rats and compare the dependence of RBC CS on transit time between healthy rats and rats exposed to a model of bronchopulmonary dysplasia (BPD). We propose possible mechanisms for this phenomenon based on changes in bulk magnetic susceptibility (BMS).Methods
10 Sprague Dawley rats were exposed to hyperoxia/hypoxia to model BPD2. 10 additional healthy Sprague Dawley rats provided a control group. Imaging was performed on 54+/-1 or 82+/-2 post natal day. Four pre-breaths of 129Xe followed by a breath hold of 129Xe were administered to the rats. The breath hold duration was kept constant for all τ, ensuring consistent oxygen saturation for all rats. During the breath-hold, a saturation pulse was applied to the dissolved phases. After waiting a delay period, τ, another saturation pulse was applied and the free-induction decay was acquired. Sensitization to 129Xe transit time in the blood was achieved by using ten different values of τ (10ms-2000ms). Three Lorentzian line shapes corresponding to the 129Xe resonances of gas, RBCs, and pulmonary tissue/plasma3 were fit to the spectra. As the frequency of inhaled 129Xe gas can vary by several ppm4, tissue/plasma was used as the reference for CS and did not vary with τ or exposure status. The CS of RBCs in the brain of healthy rats was also measured to represent an “infinite” τ.Results and Discussion
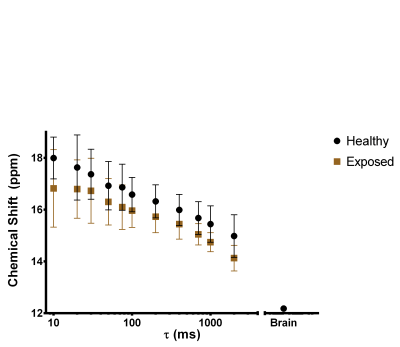
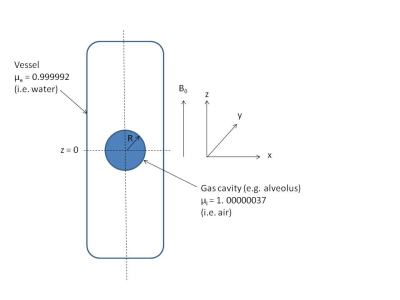
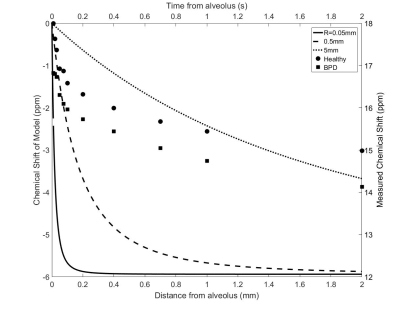
The mean CS of the RBC signal measured as a function of τ for both cohorts is shown in Fig. 1. The CS of the RBC signal decreased with increasing τ in agreement with the previously reported trend in humans1. The CS of RBC signal has been reported to increase with oxygenation5; however the oxygen saturation was constant for all τ. The change in CS of RBCs may be caused by a change in BMS as the 129Xe transits away from the gas-tissue interface at the lungs. As a first order approximation, we consider the magnetic field along the centre of a cylinder with magnetic susceptibility μe parallel to the static magnetic field surrounding a gas cavity with radius R and magnetic susceptibility μi (Fig. 2). This is given by the following equation6 and is plotted in Fig. 3 for various values of R, as a function of the distance away from the alveolus (z):
$$B_e≈B_0 (1+(μ_e-1)/3-(2(μ_e-μ_i)R^3)/(3z^3 ))$$
The shape and order of magnitude of the model is qualitatively similar to the experimental results, suggesting that the distance from the gas-tissue interface may play a role in the relationship between τ and CS. The CS of 129Xe in RBCs in the brain was -6ppm in comparison to the lung RBC CS at τ=10ms which is in good agreement with the model prediction of -5.5ppm at infinite τ.
The difference in CS between the healthy and exposed cohorts trended towards significance (two-way ANOVA, P=0.0571). It is unexpected that the difference in CS was caused by differences in oxygen saturation as it is not expected for the exposed cohort to have higher oxygen saturation. The difference may be caused by several physiological changes including increased blood flow7, increased alveolar size2, and changes to the vessel architecture8 in the BPD cohort. For an increase in blood flow, 129Xe will be further away from the gas-tissue interface for a given τ. However, the effect of τ on CS was found to be consistent for all values of τ (P=0.9838) which would not be the case if the blood velocities were different. The effect of changing gas cavity radius is shown in Fig. 3; however, the order of magnitude of the effect from microscopic changes to alveolar radius is too small to explain the measured difference and suggests a possible macroscopic BMS effect. Changes to vessel architecture cannot be captured in the simple analytical model presented here. In future, histological analysis as well as a more robust numerical simulation may be useful in order to investigate the origin of this phenomenon.
Conclusion
The CS of 129Xe dissolved in RBCs decreases as a function of transit time from the gas exchange regions of the lung in rats, similar to results previously reported in humans. Differences in the dependencies of CS on τ were also observed between the healthy rats and rats exposed to hypoxia/hyperoxia. The magnitude and polarity of the measured CS changes are consistent with the BMS difference expected at the lung air-tissue interface, which diminishes with distance from the lung as τ increases.Acknowledgements
Thanks to N. Kanhere, A. Lindenmaier, and F. Morgado for their assistance with imaging experiments. This work was supported by funding from CIHR. Y.F. was financially supported by a Queen Elizabeth II Graduate Scholarship in Science and Technology.References
1. Norquay G, Stewart NJ, Wild JM. Evaluation of 129Xe-RBC signal dynamics and chemical shift in the cardiopulmonary circuit using hyperpolarized 129Xe NMR. In: ISMRM 25th Annual Meeting. ; 2017:3327.
2. Mankouski A, Kantores C, Wong MJ, et al. Intermittent hypoxia during recovery from neonatal hyperoxic lung injury causes long-term impairment of alveolar development: A new rat model of BPD. Am J Physiol - Lung Cell Mol Physiol. 2017;312(2):L208-L216. doi:10.1152/ajplung.00463.2016.
3. Chang Y V, Quirk JD, Ruset IC, Atkinson JJ, Hersman FW, Woods JC. Quantification of Human Lung Structure and Physiology Using Hyperpolarized 129Xe. 2014;344(September 2013):339-344. doi:10.1002/mrm.24992.
4. Virgincar RS, Robertson SH, Nouls J, et al. Establishing an accurate gas phase reference frequency to quantify 129Xe chemical shifts in vivo. Magn Reson Med. 2016;77(4):1438-1445. doi:10.1002/mrm.26229.
5. Norquay G, Leung G, Stewart NJ, Wolber J, Wild JM. 129Xe chemical shift in human blood and pulmonary blood oxygenation measurement in humans using hyperpolarized 129Xe NMR. Magn Reson Med. 2016;77(4):1399-1408. doi:10.1002/mrm.26225.
6. Bhagwandien R, Moerland MA, Bakker CJG, Beersma R, Lagendijk JJW. Numerical analysis of the magnetic field for arbitrary magnetic susceptibility distributions in 3D. Magn Reson Imaging. 1994;12(1):101-107. doi:10.1016/0730-725X(94)92357-4.
7. Bancalari E, Claure N, Sosenko IR. Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition. Semin Neonatol. 2003;8(1):63-71. doi:10.1016/S1084-2756(02)00192-6.
8. Tomashefski JF, Oppermann HC, Vawter GF, Reid LM. Bronchopulmonary Dysplasia: A Morphometry Study with Emphasis on the Pulmonary Vasculature. Pediatr Pathol. 1984;2(4):469-487. doi:10.3109/15513818409025895.
Figures