0970
An integrated preclinical platform for high-resolution 3D hyperpolarized 129Xe MRI at high field1Biomedical Engineering, Duke University, Durham, NC, United States, 2Center for In Vivo Microscopy, Duke University Medical Center, Durham, NC, United States, 3Radiology, Duke University Medical Center, Durham, NC, United States
Synopsis
Hyperpolarized (HP) 129Xe MR imaging of lung function is beginning to find clinical application. This must be supported by preclinical imaging in well-characterized animal models. However, this capability is limited to a few sites, often involves only 2D projections, and is often done at non-standard, low field strengths. This work demonstrates the feasibility of preclinical 129Xe MRI at 7T, using an integrated gas delivery and physiological monitoring system and customized 3D radial acquisitions. We characterize the spectral structure of 129Xe in rats and demonstrate that despite the short T2* at high field strength, 3D gas exchange imaging should be feasible.
Introduction
Hyperpolarized (HP) 129Xe MRI has found extensive clinical application in studying lung disease 1,2. 129Xe ventilation, barrier uptake and red-blood-cell (RBC) transfer can be depicted separately and three-dimensionally by exploiting xenon solubility and large chemical shifts in different pulmonary micro-environments. Clinically, 129Xe MRI has been conducted primarily at 1.5T and is now transitioning to 3T. As clinical implementation progresses, it becomes important to translate back to well-defined animal models, where novel disease signatures can be characterized longitudinally and validated against histological ground truth. This requires 3D depiction of 129Xe in all 3 compartments (gas, barrier, RBC) to visualize localized changes in pathophysiology. This requires well-controlled HP gas administration, and respiratory-gated multi-breath acquisitions 3-5. To date, preclinical 129Xe MRI has been limited to few centers 6-9, and 3D gas exchange MRI has only been demonstrated on a custom 2T scanner 3,4. However, preclinical scanners now operate at 7T or more, and thus represent the platform on which fully integrated 129Xe gas exchange MRI must be developed to permit broader dissemination. Here we report on these efforts.Methods
Two distinct developments were implemented. A fully integrated, portable MR-compatible 129Xe mechanical ventilator was developed to enable rodents to be ventilated with non-traumatic peroral intubation. The system provides MR acquisition triggering in synchrony with the breathing cycle, and real-time physiological monitoring of heart rate and airway pressures (Figure-1).
A custom birdcage coil (70-mm inner-diameter, 100-mm long) was built to resonate at 83.06 MHz. The animal is centered in the magnet on a cantilevered bed that allows single-tuned 129Xe and 1H coils to be swapped without moving the animal. This maximizes 129Xe sensitivity and facilitates 1H/129Xe image co-registration.
In vivo imaging and spectroscopy was demonstrated on 200-g Sprague-Dawley rats, which were anesthetized with pentobarbital IP, and perorally intubated (16G catheter). Rats were ventilated with a 2-ml tidal volume (25%O2+75%N2), 1 breath/s (250-ms inspiration, 250-ms breath-hold and 500-ms exhalation). Airway pressure, ECG, and body temperature were continuously monitored (Figure-1C). Just prior to 129Xe MR acquisition, HP 129Xe replaced nitrogen by flipping a switch. Imaging consumed 350ml of isotopically-enriched 129Xe polarized to ~20%, delivered from a pressurized dose bag (Figure-1A).
129Xe gas-phase MRI was acquired using: 0.1-ms hard pulse, BW=25kHz, FOV=5cm3, 2985 total projections acquired at breath-hold, 20 projections per trigger, 32 points per projection, α=15°, TE/TR=0.056/20 ms. Each readout lasted 1.3ms to accommodate the ~2-ms gas-phase T2* estimated from spectroscopy.
129Xe dissolved-phase spectra were acquired to characterize spectral shapes, frequencies and linewidths. The excitation frequency was centered on the dissolved-phase (+17500 Hz from gas-phase) and a frequency selective 310-µs 3-lobe sinc pulse was used to suppress gas-phase excitation.
Results and Discussion
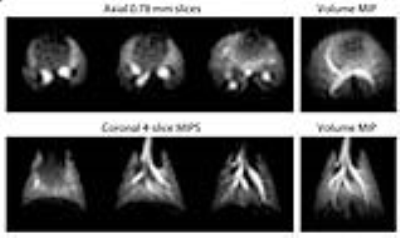
Figure-2 shows 129Xe ventilation images, acquired with 0.78-mm isotropic resolution and SNR ~80. Figure-3 shows a simplified pulse sequence, along with the end points of the uniform radial projections. Also shown is the evolution of 129Xe magnetization over the course of the acquisition, enabled by radial acquisition always sampling the k-space center. Here, the 15° flip angle induces modest signal decay over the 20 views per breath-hold, which is replenished at each inhalation. The amplitude of these signals returns to precisely the same intensity by virtue of the consistent 129Xe delivery.
Figure-4A shows the raw dissolved-phase FID and spectrum in the rat, which contains the 3 main resonances of 129Xe in the lung: gas, barrier and RBC. Figure-4B shows the 129Xe spectrum fit to a sum of 3 Lorentzians in MATLAB to extract parameters of each resonance. This revealed chemical shifts of -0.8 ppm, 195.6 ppm, and 209.9 ppm for these resonances, and linewidths of 145 Hz, 636 Hz and 419 Hz respectively. This suggests a T2* of 2.2ms in the gas-phase and a lower limit of 0.5ms for the dissolved resonances.
Figure-5 compares the 7T spectrum to a similar one acquired at 2T. While the chemical shifts are comparable, the linewidths of the gas and barrier peaks were 2.9x wider at 7T, and that of the RBC peak was 2x wider at 7T. This broadening was less than what would be predicted by the 3.5x higher field strength at 7T.
Conclusion
This work demonstrates that high-resolution 3D 129Xe imaging is feasible in rats at 7T, with an integrated ventilator and physiologic monitoring system, using a custom radial pulse sequence. Moreover, the characterization of 129Xe spectral parameters constitutes an important step to initiate full gas-exchange imaging and decomposition. Selective excitation of dissolved-phase 129Xe is possible with a sinc pulse of 310 µs, enabling dissolved imaging at TE = 213 µs, well within the 0.5-ms T2* of the shortest-lived resonance.Acknowledgements
R01HL105643, P41 EB015897References
1. Virgincar RS, Cleveland ZI, Sivaram Kaushik S, Freeman MS, Nouls J, Cofer GP, Martinez-Jimenez S, He M, Kraft M, Wolber J, Page McAdams H, Driehuys B. Quantitative analysis of hyperpolarized 129Xe ventilation imaging in healthy volunteers and subjects with chronic obstructive pulmonary disease. NMR in Biomedicine 2013;26(4):424-435.
2. Wang Z, Robertson SH, Virgincar RS, Wang J, Bier E, He M, Driehuys B. Quantitative Gas Exchange using Hyperpolarized 129Xe MRI in Idiopathic Pulmonary Fibrosis. Proc Intl Soc Mag Reson Med 2016;1628.
3. Robertson SH, Virgincar RS, He M, Freeman MS, Kaushik SS, Driehuys B. Optimizing 3D noncartesian gridding reconstruction for hyperpolarized 129Xe MRI—focus on preclinical applications. Concepts in Magnetic Resonance Part A 2015;44(4):190-202.
4. Cleveland ZI, Virgincar RS, Qi Y, Robertson SH, Degan S, Driehuys B. 3D MRI of impaired hyperpolarized Xe-129 uptake in a rat model of pulmonary fibrosis. Nmr Biomed 2014;27(12):1502-1514.
5. Nouls J, Fanarjian M, Hedlund L, Driehuys B. A Constant-Volume Ventilator and Gas Recapture System for Hyperpolarized Gas MRI of Mouse and Rat Lungs. Concepts Magn Reson Part B 2011;39B(2):78-88.
6. Ruan W, Zhong J, Wang K, Wu G, Han Y, Sun X, Ye C, Zhou X. Detection of the mild emphysema by quantification of lung respiratory airways with hyperpolarized xenon diffusion MRI. Journal of Magnetic Resonance Imaging 2017;45(3):879-888.
7. Iguchi S, Imai H, Hori Y, Nakajima J, Kimura A, Fujiwara H. Direct imaging of hyperpolarized 129Xe alveolar gas uptake in a mouse model of emphysema. Magnetic Resonance in Medicine 2013;70(1):207-215.
8. Doganay O, Wade T, Hegarty E, McKenzie C, Schulte RF, Santyr GE. Hyperpolarized 129Xe imaging of the rat lung using spiral IDEAL. Magnetic Resonance in Medicine 2016;76(2):566-576.
9. Branca RT, He T, Le Z, Floyd CS, Freeman M, White C, Burant A. Detection of brown adipose tissue and thermogenic activity in mice by hyperpolarized xenon MRI. Proceedings of the National Academy of Sciences of the United States of America 2014;111(50):18001-18006.
Figures