0966
Hyperpolarized 129Xe MR spectroscopy detects short-term changes in lung gas exchange efficiency in idiopathic pulmonary fibrosis1University of Sheffield, Sheffield, United Kingdom, 2Sheffield Teaching Hospitals, Sheffield, United Kingdom, 3Sheffield Children's Hospital NHS Foundation Trust, Sheffield, United Kingdom
Synopsis
Idiopathic pulmonary fibrosis (IPF), once thought of as an orphan lung disease, is now a frontrunner in respiratory research. However, progress is hampered by a lack of sensitive biomarkers. Hyperpolarized xenon MR spectroscopy has demonstrated sensitivity to gas exchange in IPF and is emerging as a feasible imaging biomarker of disease. Here, we demonstrate that this methodology has high reproducibility in the disease state, correlates with clinical outcomes and demonstrates a decline in gas transfer efficiency in the lung over six months in spite of static pulmonary function test metrics.
Motivation
Recent advances in magnetic resonance spectroscopy (MRS) have permitted the use of inhaled hyperpolarized xenon (129Xe) to assess the alveolar gas exchange interface.1-3 Xenon is soluble in lung parenchyma and red blood cells and the chemical shifts that accompany these changes in chemical environment allow for quantitative assessment of the acinar gas exchange efficiency.4 This has been examined most closely in idiopathic pulmonary fibrosis (IPF), a commonly fatal disease of lung scarring.5 However, to develop 129Xe spectroscopy as a new biomarker, sensitivity to change must be longitudinally assessed. Herein, we assess six- and twelve-month changes in 129Xe MRS metrics in a cohort of participants with IPF.Methods
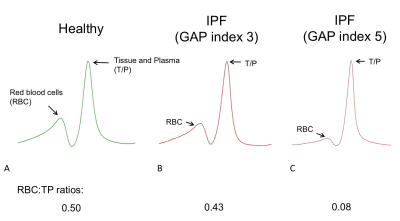
In a prospective study, participants with a new multidisciplinary diagnosis of IPF underwent hyperpolarized 129Xe lung MR spectroscopy. Ten volunteered to undergo a further scan on the same day to assess reproducibility. Further MR assessments took place 6 and 12 months after baseline scans. 600mL of 129Xe was mixed with nitrogen to balance a total inhaled dose of 1L. MR spectroscopy data was acquired at 1.5T during a 15 second breath-hold after inhaling the gas mixture from a lung volume of functional residual capacity. A high-resolution MR spectroscopy sequence was used to acquire MR spectra of 129Xe dissolved in the lung tissue and plasma (TP) and red blood cell (RBC) compartments. After phasing the acquired MR spectra, integrals of the RBC and TP spectral peaks were evaluated and expressed as the ratio RBC:TP (see Figure 1). Pulmonary function tests (PFTs) were performed on the same day, including spirometry for forced vital capacity (FVC) and diffusing capacity / coefficient of the lung for carbon monoxide (DLCO / KCO). Spearman’s rho, intra-class correlation (ICC) and Wilcoxon Rank test was used to determine the strength of correlations, reproducibility and the significance of differences in baseline and six-month metrics, respectively.Results
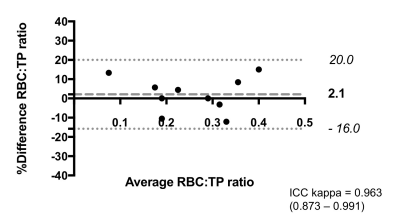
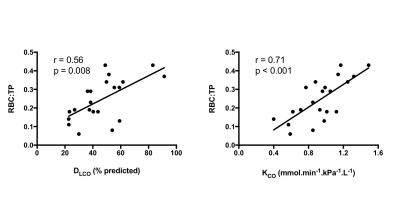
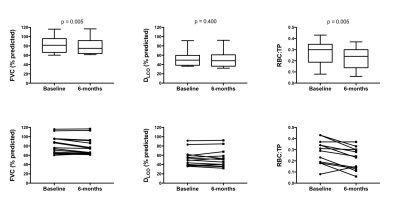
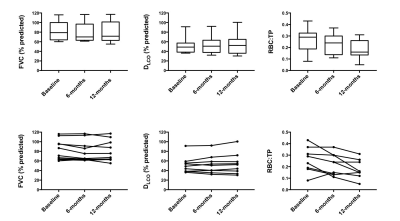
MRS scans were well tolerated in all participants. RBC:TP was highly reproducible with an ICC 0.96 (see Figure 2) confirming previous preliminary results.6 Fourteen participants returned at six months (mean: 195 days; standard deviation: 24 days); four participants died prior to follow up and three more are due to return before the end of 2017. At the time of writing, 9 participants have returned for 12-month scans (mean: 352 days; standard deviation: 24 days). Example 129Xe MR spectra from a healthy subject and two IPF patients with different disease severities are provided in Figure 1. Significant correlations were found between baseline RBC:TP and DLCO (r=0.56; p=0.008) and KCO (r=0.71; p<0.001) but not with FVC (r=0.18; p=0.432), as shown in Figure 3. Correlations remained significant at six months for RBC:TP with DLCO (r=0.831; p<0.001), but not KCO (r=0.37; p=0.192). Six-month changes (median percentage change; Wilcoxon p-value) were statistically significant for FVC (-4.8%; p=0.005) and RBC:TP (-20.5%; p=0.005), but not for DLCO (-4.4%; p=0.400) or KCO (-1.8%; p=0.143), as shown in Figure 4. Percentage change in 129Xe RBC:TP was not significantly correlated with percentage change in FVC (r=0.511; p=0.064), DLCO (r=-0.419; p=0.137) or KCO (r=0.425; p=0.130).Discussion
In keeping with previous studies,1,2 our results demonstrate sensitivity of 129Xe MRS to the gas exchange efficiency of the lung in IPF. RBC:TP was found to be highly reproducible in participants with IPF. The lack of sensitive, robust biomarkers for IPF assessment make novel techniques to assess the underlying pathophysiology appealing.7 Thus, the fact that the decline in RBC:TP demonstrated in our study was not accompanied by a change in DLCO or KCO is of significant interest. This may be due to differences in gas properties, posture or technique. DLCO is calculated from exhaled gas concentration at the mouth and thus does not provide regional information on gas exchange. In contrast, MR spectroscopy assesses the gas exchange properties of the lung by obtaining measurements directly from the alveoli, alveolar-capillary membranes and RBCs. There is no definitive way of ensuring that physiological decline is represented by the fall in RBC:TP in this small cohort. However, the death of four participants with low baseline RBC:TP and the associated – but not correlated – decline in FVC suggests that 129Xe MRS may provide an opportunity to measure physiological decline which is not well represented by current clinical metrics of lung function in IPF. We anticipate the availability of sufficient 12-month data for accurate statistical analysis by the time of the meeting and early indications (n=9) show this to be supportive of further RBC:TP decline in this population (Figure 5).Acknowledgements
This work was supported by NIHR grant NIHR-RP-R3-12-027 and MRC grant MR/M008894/1. The views expressed in this work are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.References
1. Stewart NJ, et al. Magn Reson Med. 2015;74(1):196-207.
2. Kaushik SS et al. J Appl Physiol. 2014;117(6):577-85.
3. Wang Z et al. Med Phys. 2017:06.
4. Driehuys B et al. Proc Natl Acad Sci USA. 2006;103(48):18278-83.
5. Raghu G, et al. Am J Resp Crit Care Med. 2011;183(6):788-824.
6. Stewart NJ, et al. Proc Intl Soc Mag Reson Med. 25 (2017): 2148.
7. Wells AU. Thorax. 2013;68(4):309-10.
Figures