0964
Hyperpolarized 129Xe Multiple Breath Washout MRI in Pediatric Cystic Fibrosis1Translational Medicine, The Hospital for Sick Children, Toronto, ON, Canada, 2Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 3Division of Respiratory Medicine, The Hospital for Sick Children, Toronto, ON, Canada
Synopsis
The lung clearance index, measured using N2 multiple breath washout (MBW), provides an indicator of ventilation heterogeneity but lacks regional information. The combination of MBW and hyperpolarized 129Xe MRI can potentially provide measurements of ventilation heterogeneity that include both spatial and temporal information. MBW imaging was performed following an initial 129Xe inhalation and during multiple breath-holds of room air to measure the 129Xe washout. Fractional ventilation and coefficient of variation maps measured in pediatric cystic fibrosis participants show elevated ventilation heterogeneity compared to age-matched healthy controls.
Introduction
Multiple breath washout (MBW) is a test that can be used to derive the lung clearance index (LCI), which is sensitive to ventilation heterogeneity and early lung disease.1 Although LCI is more sensitive than clinically used spirometric indices, such as the forced expiratory volume in one second (FEV1),2 both tests provide whole-lung measurements that cannot identify the specific lung regions that contribute to the ventilation heterogeneity. Hyperpolarized (HP) 129Xe MRI is a lung imaging technique that is feasible in children with CF,3 and images can be quantified using the ventilation defect percent (VDP).4 The combination of MBW with HP gas imaging can potentially provide both temporal and spatial washout information.5 MBW imaging has been performed in pediatric CF using HP 3He,6 but the feasibility of HP 129Xe MBW imaging has not yet been demonstrated in a pediatric population. The purpose of this study was to perform HP 129Xe MBW MRI in pediatric CF, and to compare it to LCI and FEV1.Methods
Eleven pediatric participants were recruited (mean age 11.4±2.4 years), including four with well-controlled CF and seven aged-matched healthy controls. Standard pulmonary function tests were performed, as well as N2 MBW (Exhalyzer D®, EcoMedics AG) to measure LCI (2.5% threshold). Imaging was performed at 3T (Siemens Prisma) using a flexible 129Xe vest coil (Clinical MR Solutions). 129Xe was polarized (Polarean, Durham, NC) and dosed at 10% of total lung capacity (balanced with N2 in a 1L dose bag). Following the method of Horn et al.,5 the 129Xe dose was inhaled from functional residual capacity, and two 129Xe images (separated by a 5s delay) were acquired to correct for RF- and T1-induced signal decay. The participant exhaled, inhaled room air, held their breath, and repeated this procedure as a series of 4–6 washout images were acquired. Each image was a 2D projection acquired in the coronal plane (gradient echo, TR=8.1ms, TE=1.9ms, FA=4°, FOV=480x480mm2, matrix=64x64, and BW=100Hz/pixel). Fractional ventilation, r, defined as the fractional gas replacement per breath, was calculated using previously described MBW signal equations.5 To assess ventilation heterogeneity, coefficient of variation (CV) maps were calculated from the r maps.7Results
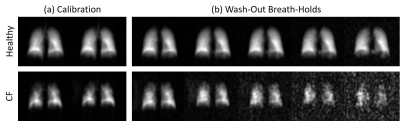
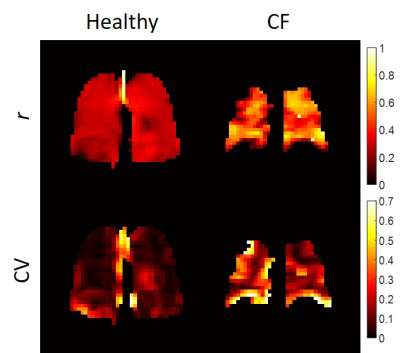
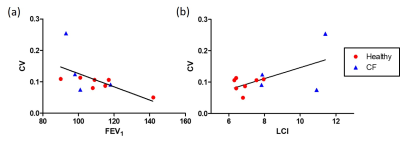
There was a statistically significant difference in LCI between the CF and healthy groups (9.5±1.9 and 6.9±0.6, respectively, p=0.02 from a Wilcoxon rank-sum test), but FEV1 was not statistically different (103±11% and 112±16%, respectively, p=0.22). Figure 1 shows two representative series of HP 129Xe MBW images acquired in a healthy participant and in a CF patient. Corresponding r and CV maps calculated from Fig. 1 are shown in Fig. 2. CF participants had a mean r of 0.51±0.07 while healthy participants had a mean r of 0.42±0.11 (p=0.07). r was not correlated with FEV1 (R2=4.1x10-5), but it may be very weakly correlated with LCI (R2=0.15). CF participants had a mean CV of 0.14±0.08 and healthy participants had a mean CV of 0.09±0.02. Although the differences in CV were not significant between groups (p=0.51), there was a weak correlation between CV and both FEV1 (R2=0.34) and LCI (R2=0.34) (Fig. 3).Discussion
There was a trend in this study towards increasing mean r in CF subjects compared to healthy controls, which was an unexpected result given the increase in both VDP and LCI previously measured in a similar pediatric population.8 One possible explanation for this finding is that HP 129Xe MBW imaging uses a single breath washin prior to imaging. Therefore, the 129Xe gas may not have been able to fully equilibrate in the most obstructed areas of the lung before the washout breaths. Therefore, MBW imaging results may represent the washout kinetics of better-ventilated regions of the lung, contributing to higher observed r in CF lungs that have overall worse ventilation heterogeneity.
This study also showed a trend towards increased CV in CF, and a weak correlation between CV and both FEV1 and LCI. Therefore, CV may be more reflective of the underlying physiologic abnormalities seen in CF than r alone. There were a few limitations in this study, such as the use of 2D projection imaging, which may cause partial volume artifacts that add additional weighting to r maps. In addition, this study assumed a constant 5s delay, constant tidal volume, and constant T1 for each washout breath; therefore, poor subject compliance and changes in T1 due to an increasing oxygen partial pressure with each washout breath will also bias r maps.
Conclusions
This study demonstrates that HP 129Xe MBW imaging is feasible in pediatric participants with CF. Mapping ventilation heterogeneity using HP 129Xe MRI is expected to provide a useful tool for detecting functional changes that result from both disease progression and treatment response in early CF lung disease.Acknowledgements
We thank Tammy Rayner and Ruth Weiss for assistance with MRI data acquisition. This work was funded in part by a Cystic Fibrosis Research Catalyst from The Hospital for Sick Children, a Canadian Institutes for Health Research (CIHR) operating grant (MOP 123431), and a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery grant (RGPIN 217015-2013). MJC was funded by a Restracomp fellowship award from The Hospital for Sick Children, and a Mitacs Elevate postdoctoral fellowship.References
1. Gustafsson PM, Aurora P, Lindblad A. Evaluation of ventilation maldistribution as an early indicator of lung disease in children with cystic fibrosis. Eur Respir J 2003;22(6):972-979.
2. Aurora P, Bush A, Gustafsson P, Oliver C, Wallis C, Price J, Stroobant J, Carr S, Stocks J. Multiple-breath washout as a marker of lung disease in preschool children with cystic fibrosis. Am J Respir Crit Care Med 2005;171(3):249-256.
3. Walkup LL, Thomen RP, Akinyi TG, Watters E, Ruppert K, Clancy JP, Woods JC, Cleveland ZI. Feasibility, tolerability and safety of pediatric hyperpolarized 129Xe magnetic resonance imaging in healthy volunteers and children with cystic fibrosis. Pediatr Radiol 2016;46(12):1651-1662.
4. Thomen RP, Walkup LL, Roach DJ, Cleveland ZI, Clancy JP, Woods JC. Hyperpolarized 129Xe for investigation of mild cystic fibrosis lung disease in pediatric patients. J Cyst Fibros 2017;16(2):275-282.
5. Horn FC, Deppe MH, Marshall H, Parra-Robles J, Wild JM. Quantification of regional fractional ventilation in human subjects by measurement of hyperpolarized 3He washout with 2D and 3D MRI. J Appl Physiol 2014;116(2):129-139.
6. Horn FC, Marshall H, Siddiqui S, Horsley A, Smith L, Aldag I, Kay R, Taylor CJ, Parra-Robles J, Wild JM. Ventilation heterogeneity in obstructive airways disease – comparing multi-breath washout-imaging with global lung measurements. Proc ISMRM 2015;23:852.
7. Tzeng YS, Lutchen K, Albert M. The difference in ventilation heterogeneity between asthmatic and healthy subjects quantified using hyperpolarized 3He MRI. J Appl Physiol 2009;106:813-822.
8. Kanhere N, Couch MJ, Kowalik K, Zanette B, Rayment JH, Manson D, Subbarao P, Ratjen F, Santyr G. Correlation of Lung Clearance Index with Hyperpolarized 129Xe Magnetic Resonance Imaging in Pediatric Subjects with Cystic Fibrosis. Am J Respir Crit Care Med 2017;196(8):1073-1075.
Figures