0960
Radiomics utilizing Fractional Anisotropy in Peritumoral Nonenhancing Region Predicts Local Progression and Overall Survival in Patients with Glioblastoma1Laboratory for Imaging Science and Technology, Department of Electrical and Computer Engineering, Seoul National University, Seoul, Republic of Korea, 2Department of Radiology and Research Institute of Radiology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Republic of Korea
Synopsis
We explored the radiomic features of peritumoral nonenhancing lesion in newly diagnosed glioblastoma patients to predict local progression and overall survival using fractional anisotropy (FA) at 3 Tesla. Among 1618 extracted radiomic features, 8 FA features were significantly associated with 6-month progression and overall survival (OS). The cross-validated area under the ROC curve (AUC) for 6-month progression was 0.71 and C-index for OS was 0.75. FA radiomics in nonenhancing lesion has the potential for predicting local progression and overall survival in glioblastoma.
Target audience
Researchers and clinicians who
are interested in radiomic applications, DTI, and brain gliomas.Purpose
Glioblastomas are diffusely infiltrating tumors, and a widely accepted concept is that the margins of the contrast-enhancing region do not represent the true tumor margins (1). Most of the peritumoral nonenhancing region (NER) left behind during surgical resection and most recurrences may occur within the treatment field (2, 3). Diffusion tensor imaging is an important tool for preoperative planning, which provides quantitative information including fractional anisotropy (FA). Recent studies showed that reduced FA in regions adjacent to glioblastomas significantly associated with tumor infiltration (4) and future recurrence (5). However, the FA analysis in NER is complicated due to different tumor locations and adjacent white matter structure. A recently introduced radiomics model extracts descriptors using an automated data mining algorithm and extends magnetic resonance imaging data into a high-dimensional feature space (6, 7). The relationship between voxels can be derived from the high-throughput imaging features, and may prone to discover hidden information inaccessible with single parameter approach. We investigated if FA-based radiomics analysis in NER predicts local recurrence and overall survival in patients with glioblastoma.Materials and Methods
This retrospective study was approved by our institutional review board. This retrospective study included 83 patients with newly diagnosed glioblastomas. The patients were obtained with contrast-enhanced T1-weighted imaging (T1-CE), fluid-attenuated inversion recovery (FLAIR), and DTI. The DTI was performed with b values of 600 sec/mm2 and 0 sec/mm2, 32 directions, and the following parameters: TR/TE 8413.4/77; field of view, 220 mm; section thickness, 2 mm; and matrix, 112 × 112 on the 3.0 Tesla unit (Achieva; Philips Medical Systems, Best, The Netherlands). All diffusion tensor imaging (DTI) data was analyzed using toolboxes in FMRIB Software Library (FSL) software package version 5.0.8 (http://www.fmrib.ox.ac.uk/fsl/, FMIRB Analysis Group, Oxford, UK). After the rigid body registration, the transformation matrix was applied to FA map using FMRIB’s Linear Image Registration Tool (FLIRT). Radiomics features were extracted from FA maps, in the NER region drawn using in-house Matlab (R2014b). Total 4854 features (1618 features from each imaging, including 17 first-order, 7 volume and shape, 162 texture, and 1432 wavelet features) were extracted from T1CE, FLAIR, and FA maps. The local progression was defined as 6-month progression on the imaging follow-up. Overall survival was calculated from the date of diagnosis to the death of any cause obtained from the national health insurance database. Least absolute shrinkage and selection operator (LASSO) model was applied to select features to predict 6-month progression and overall survival, respectively. The area under the receiver operating characteristics curve (AUC) tested to predict 6-month progression. Cox hazards model-based performance was assessed with C index to predict, and compared with model with established clinical parameters comprising age, sex, Karnofsky performance score, and surgical extent with 10 fold cross-validation.Results and Discussion
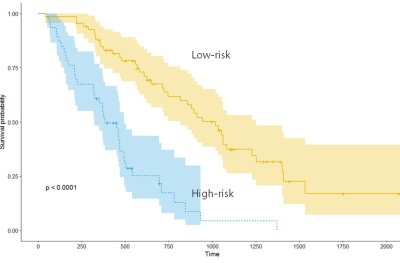
Eight high-ranking FA radiomic features (all second-order features) (Table 1) were selected using LASSO (Figure 1). No significant features were extracted from T1CE and FLAIR imaging. The FA radiomic features stratified patients into a longer- and shorter survival group in both training and test set (log rank test, both P <.0001) (Figure 2). In diagnosing 6-month progression, the FA radiomics result showed AUC 0.71, For predicting OS, the performance of radiomics model (C- index, 0.75) was better than that of the established clinical model (C index, 0.62). Our results are consistent with a recent study by Bette et al (5), who reported that reduced local FA significantly associated with patients with local recurrence in glioblastomas.Conclusion
The FA radiomics in the NER has potential to predict local progression and overall survival, whereas T1CE and FLAIR based radiomics do not. Non-invasive prediction of local progression and overall survival with preoperative FA could provide useful information in patients with newly diagnosed glioblastoma.Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (grant number: NRF-2017R1C1B2007258).References
1. Stummer W, Reulen HJ, Meinel T, Pichlmeier U, Schumacher W, Tonn JC, et al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62(3):564-76; discussion -76.
2. Dobelbower MC, Burnett Iii OL, Nordal RA, Nabors LB, Markert JM, Hyatt MD, et al. Patterns of failure for glioblastoma multiforme following concurrent radiation and temozolomide. J Med Imaging Radiat Oncol. 2011;55(1):77-81.
3. Jain R, Poisson LM, Gutman D, Scarpace L, Hwang SN, Holder CA, et al. Outcome prediction in patients with glioblastoma by using imaging, clinical, and genomic biomarkers: focus on the nonenhancing component of the tumor. Radiology. 2014;272(2):484-93.
4. Min ZG, Niu C, Rana N, Ji HM, Zhang M. Differentiation of pure vasogenic edema and tumor-infiltrated edema in patients with peritumoral edema by analyzing the relationship of axial and radial diffusivities on 3.0T MRI. Clin Neurol Neurosurg. 2013;115(8):1366-70.
5. Bette S, Huber T, Gempt J, Boeckh-Behrens T, Wiestler B, Kehl V, et al. Local Fractional Anisotropy Is Reduced in Areas with Tumor Recurrence in Glioblastoma. Radiology. 2017;283(2):499-507.
6. Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nature communications. 2014;5:4006.
7. Kumar V, Gu Y, Basu S, Berglund A, Eschrich SA, Schabath MB, et al. Radiomics: the process and the challenges. Magnetic resonance imaging. 2012;30(9):1234-48.
Figures