0956
Magnetic Resonance Spectroscopy markers of survival in paediatric brain tumours: A 3T Multi-Centre investigation1University of Birmingham, Birmingham, United Kingdom, 2Birmingham children's hospital, Birmingham, United Kingdom, 3Department of Radiology, Alder Hey Children's NHS Foundation Trust, Liverpool, United Kingdom, 4Institute of Digital Healthcare, WMG, University of Warwick, Coventry, United Kingdom, 5Paediatric Oncology Department, Great North Children’s Hospital, Newcastle upon Tyne, United Kingdom, 6Department of Imaging and Medical Physics, University Hospitals Birmingham NHS Foundation Trust, Birmingham, United Kingdom, 7The Children‘s Brain Tumour Research Centre, University of Nottingham, Nottingham, United Kingdom, 8Radiological Sciences, Department of Clinical Neuroscience, University of Nottingham, Nottingham, United Kingdom, 9Medical Physics, Nottingham University Hospital, Queen’s Medical Centre, Nottingham, United Kingdom, 10Neuroradiology, Nottingham University Hospital, Queen’s Medical Centre, Nottingham, United Kingdom, 11Neuroradiology Department, Newcastle upon Tyne Hospitals, Newcastle upon Tyne, United Kingdom, 12Department of Paediatric Oncology, Alder Hey Children's NHS Foundation Trust, Liverpool, United Kingdom
Synopsis
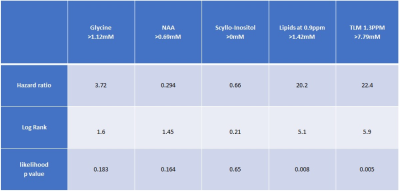
Brain tumours have a high mortality rate and are the most common solid tumour of childhood. The non-invasive imaging technique, MRS, measures tumour metabolites which can provide additional prognostic information to aid in clinical management. MRS metabolites Glycine, Scyllo-Inositol, NAA and Lipids have been associated with prognosis for pediatric brain tumour patients in a single centre 1.5T study. This study aimed to validate these MRS survival markers in a 3T multicentre setting. In this preliminary study Lipids were validated as a survival marker across childhood brain tumours.
PURPOSE:
Brain tumours have a high mortality rate and are the most common solid tumour of childhood [1]. Identification of high risk patients may allow for better treatment stratification and more effective disease management, increasing survival and reducing treatment related damage. Magnetic Resonance Spectroscopy (MRS) provides a non-invasive measure of brain tumour metabolism[2], which has been previously used to identify diagnostic and prognostic metabolite markers in adult and paediatric brain tumours[3]. Metabolites such as high lipids, scyllo-inositol (SI), and glycine (Gly) have been shown to reflect poor survival, whilst N-Acetylaspartic acid (NAA) is a marker of good outcome in paediatric brain tumours[4, 5, 6]. However, studies have focussed on 1.5T spectroscopy and are based on single centre studies. A study at higher field strengths would increase spectral resolution and SNR therefore distinguish more metabolites. To date, metabolites measured by MRS have not been investigated as a potential survival marker in a multi-centre study for paediatric brain tumours. The aim of this preliminary study were to investigate if the established 1.5T single centre survival markers hold true in a 3T multi-centre setting.METHODS:
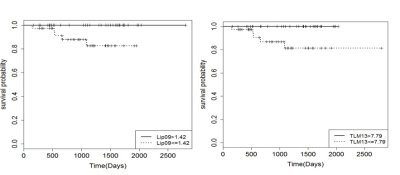
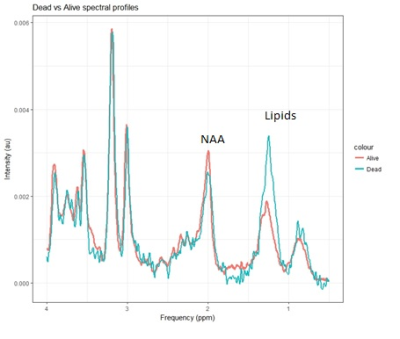
RESULTS and DISCUSSION:
Conclusion:
Lipids have been found to be a predictor of survival for childhood brain tumours in a multicentre setting, adding confidence to using MRS for predicting prognosis in different centres.Acknowledgements
We would like to thank Dr Paul Davies for advice on the statistical methodology and analysis.References
[1] Bleyer W et al. 8 drugs in 1 day chemotherapy for brain tumors: a new approach and rationale for preradiation chemotherapy. Med Pediat Oncol: 1983 11: 213
[2] Peet AC, Arvanitis TN, Auer DP, Davies NP, Hargrave D, and F. Howe, The value of magnetic resonance spectroscopy in tumour imaging. Arch Dis Child, 2008. 93: p. 725-727.
[3] Verma A, Kumar I, Verma N, Aggarwal P, and R. Ojha, Magnetic resonance spectroscopy — Revisiting the biochemical and molecular milieu of brain tumours. BBA Clinical, 2016. 5: p. 170-178.
[4] Wilson M, Cummins CL, and MacPherson LM et al, Magnetic resonance spectroscopy metabolite profiles predict survival in paediatric brain tumours. Eur. J. Cancer, 2013. 49: p. 457-464.
[5] Wilson M, Gill SK, Macpherson L, English M, Arvanitis TN, and A. Peet, Noninvasive detection of glutamate predicts survival in paediatric medulloblastoma. Clin Cancer Res, 2014. 20(17): p. 4532-4539.
[6] B.Babourina-Brooks, S.Kohe, Gill SK, Wilson M, MacPherson L, N.P.Davies, A.C.Peet. Glycine a non-invasive marker of survival in paediatric brain tumours. International Society for Magnetic Resonance in Medicine (ISMRM), Honolulu,USA, 2017. Abstract1102
[7] Wilson M. et al. A constrained least-squares approach to the automated quantitation of in vivo ¹H magnetic resonance spectroscopy data. Mag Reson Med 2011;65:1-12
[8] Murphy PS, Rowland IJ, Viviers L, Brada M, Leach MO, and Dzik-Juras AS, Could assessment of glioma methylene lipid resonance by in vivo (1)H-MRS be of clinical value? Br J Radiol, 2003. 76(907): p. 459-463.
[9] Negendank W, Li CW, Padavic-Shaller K, Murphy-Boesch J, and Brown TR, Phospholipid metabolies in 1H-decoupled 31P MRS in vivo in human cancer: implications for experimental models and clinical studies. Anticancer Res, 1996. 16(3B): p. 1539-1544.
[10] Kaminogo M, Ishimaru H, and Morikawa M et al, Diagnostic potential of short echo time MR spectroscopy of gliomas with single-voxel and point-resolved spatially localised proton spectroscopy of brain. Neuroradiology, 2001. 43(5): p. 353-363.
[11] Opstad KS, Bell BA, Griffiths JR, and Howe FA, An investigation of human brain tumour lipids by high-resolution magic angle spinning 1H MRS and histological analysis. NMR Biomed, 2008. 21(7): p. 677-685.
Figures