0953
Selective Size Imaging using Filters via diffusion Times (SSIFT): A new contrast-free highly-specific MR cancer imaging method1radiology and radiological sciences, Vanderbilt University Medical Center, Nashville, TN, United States, 2Radiation Oncology, Vanderbilt University Medical Center, Nashville, TN, United States, 3Biostatistics, Vanderbilt University Medical Center, Nashville, TN, United States
Synopsis
We propose a novel, exogenous-agent-free, highly-specific, and high-resolution cancer imaging technique termed SSIFT. Based on different diffusion time dependence on length scales, SSIFT creates filters via appropriately chosen diffusion times to selectively enhance detection sensitivity to cancer cells with simultaneous suppression of sensitivity to normal brain cells, vasogenic edema, and cystic fluid. In the first applications in metastatic brain cancer patients, SSIFT is capable of significantly enhancing tumor conspicuity and delineation, and more importantly capable of differentiating tumor recurrence from radionecrosis, which is not reliably achievable by current MRI methods.
Introduction
MRI with gadolinium (Gd)-based contrast agents (Gd-MRI) is the current clinical standard-of-care for brain cancer imaging. However, Gd-MRI has two major disadvantages: [i] Gd is not safe for patients with renal dysfunction; and [ii] Gd-MRI cannot differentiate recurrent tumors from other brain etiologies with disrupted blood brain barrier (BBB), such as radionecrosis. The latter makes post-treatment management of patients extremely challenging, and there is an urgent clinical need to develop a reliable Gd-free imaging method to differentiate tumor recurrence from radionecrosis. In the current work, we introduce a novel diffusion MRI (DWI) technique termed Selective Size Imaging using Filters via diffusion Times (SSIFT) which selectively suppresses sensitivity to normal tissues, vasogenic edema, or cystic fluid and provides a specific detection of tumors. We also report the first clinical application of SSIFT in metastatic brain cancer patients.Theory
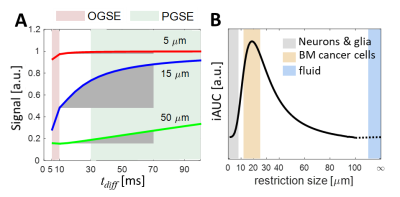
The detection sensitivity of DWI depends on the diffusion time $$$t_{diff}$$$, which in turn provides an opportunity to tune detection sensitivity to different length scales1. If we use both conventional pulsed gradient spin echo (PGSE) and oscillating gradient spin echo (OGSE) DWI, a much broader range of $$$t_{diff}$$$ can be achieved. Fig.1A shows the calculated dependence of DWI signals on three representative restriction sizes. The incremental area under curve (iAUC) shows strong dependence on cell size. In the $$$t_{diff}$$$ range of 10 to 70 ms, large cancer cells dominate time dependence compared to e.g. small dendrites and axons and free water as shown in Fig.1B. By contrast, normal brain cells are relatively much smaller. Therefore, an appropriately tuned $$$t_{diff}$$$ range may serve as a filter to selectively enhance detection sensitivity to cancer cells with simultaneous suppression of sensitivity to normal brain cells, vasogenic edema, and cystic fluid. This forms the biophysical basis of the SSIFT method.Methods
Acquisition:
Three pre- and two post-radiosurgery metastatic brain cancer patients participated in this study. Imaging was performed on a 3T whole body Philips scanner (Philips Achieva, Best, Netherlands) using a 32-channel head coil. DWI was acquired with an isotropic 2 mm resolution, TR/TE = 15 s/ 118 ms, NEX = 1, 32 diffusion directions, and b = 1000 s/mm2. Two diffusion times were used: PGSE sequence used Δ = 74 ms, δ = 12 ms while OGSE used δ = 40 ms, number of cycles = 1, resulting two effective diffusion times 70 and 10 ms, respectively. For comparison, clinical standard contrast-enhanced anatomical T1w and T2 FLAIR volumes were acquired.
Data analysis:
All diffusion images were pre-processed using FSL and MRtrix3. The average of signals over all diffusion directions of the same b value was used to remove diffusion anisotropy influence2. By normalizing spherically averaged diffusion signals using b=0 signals of cerebrospinal fluid (CSF) to remove influences of inter-scan variations (e.g., changes in receiver gain), the incremental area under curve (iAUC, see Fig.1) was obtained. In addition, DTI metrics i.e., FA and ADC, were also obtained for comparison.
Results
Conspicuity and delineation of metastatic brain tumor
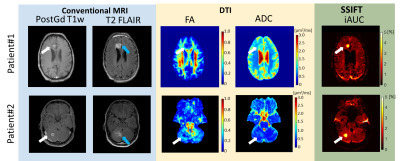
Fig.2 shows multiple parametric images of two pre-surgery cancer patients. The normalized iAUC from SSIFT provides an excellent delineation of the tumor by suppressing sensitivity to background brain tissues, including peri-tumor vasogentic edema which often confounds the detection of tumors using conventional Gd-free MRI methods. SSIFT is much less sensitive to edema, providing a possible means to identify the precise tumor boundary.
Differentiation of recurrent tumor from radionecrosis
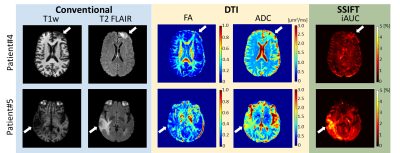
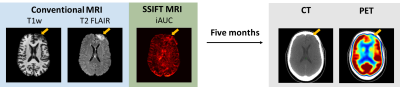
Fig.3 shows multi-parametric images of two cancer patients several months after radiation therapy. It is extremely challenging for conventional MRI T1w, T2 FLAIR, and DTI to distinguish the different responses of two patients. By contrast, SSIFT suggests the lesion of patient#4 (top) is likely radionecrosis while patient#5 (bottom) contains a significant amount of cancer cells in the lesion. Five months later, PET/CT shows “decreased metabolic activity LEFT frontal lobe … with no hypemetabolic tumor identified” in patient#4 see Fig.4). A neuropathology report also confirms “metastatic poorly differentiated carcinoma” in patient#5.
Conclusion and Discussion
We propose a novel, exogenous-agent-free, highly-specific, and high-resolution cancer imaging technique termed SSIFT for cancer imaging. In the first applications in metastatic brain cancer patients, SSIFT is capable of enhancing tumor conspicuity and delineation, and more importantly capable of differentiating tumor recurrence from radionecrosis, which is not reliably achievable by current MRI methods. By integrating emerging techniques such as multi-band, we have already reduced the total scan time of SSIFT to < 10 mins with a high isotropic resolution of 1.5 mm. This in turn makes SSIFT feasible as a sensitive and specific cancer imaging method in clinical practice.Acknowledgements
The authors thank Drs. Hua Li, Yansong Zhao, and Ke Li for assistance in pulse sequence optimization and stimulating discussion, Drs. Christien Kluwe and Nitesh Rana for assistance in coordinating studies, and MR technologists Clair Kurtenbach, Leslie McIntosh and Chris Thompson for assistance with data acquisition and subject interaction.References
1 Gore, JC et al., NMR Biomed 2010; 23: 745-756.
2 Kaden, E et al., Magn Reson Med 2016;75(4):1752-1763.
Figures