0945
MRI Compatible Hypobaric Chamber to study Early Aeromedical Evacuation Following Traumatic Brain InjurySu Xu1, Sijia Guo1, Steven Roys1, Julie L Proctor2, Gary Fiskum2, and Rao Gullapalli1
1Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States, 2Department of Anesthesiology and the Center for Shock Trauma and Anesthesiology Research, University of Maryland School of Medicine, Baltimore, MD, United States
Synopsis
Combat-related TBI leads to significant mortality and morbidity. In our previous research, we have demonstrated that simulated aeromedical evacuation (AE), or hypobaria, worsens neurological outcomes after TBI and suggests that early AE of TBI patients contributes to secondary insults. To study the effects of such hypobaric exposure during early stage of injury we have constructed a MRI compatible hypobaric chamber that allows us to evaluate changes in metabolism, perfusion, and functional status in a preclinical model of brain injury. Here we demonstrate our initial experiences with the hypobaric chamber in the MRI to obtain in vivo imaging and spectroscopic data.
Introduction
There is increased evidence that exposure to evacuation relevant hypobaric conditions worsens neurological outcomes and potentially exacerbates secondary injury1. We demonstrated that rats exposed to underbody blasts and then to hypobaria under 100% O2 exhibit increased axonal damage and impaired motor function compared to those subjected to blast and hypobaria under normoxic conditions2. In another study we demonstrated significantly worsened cognitive deficits, hippocampal neuronal loss, and microglial/astrocyte activation when rats were exposed to hypobaria following fluid percussion injury3. These findings compelled us to closely examine the effects of hypobaria in the brain in vivo during a flight by simulating the experiences of a war-fighter being evacuated soon after injury which led us to the construction of a MRI compatible hypobaric chamber.Methods – Hypobaric Chamber Construction
The hypobaric chamber was constructed by maximizing the utility of the standard rat bed on a 7.0 Tesla scanner from Bruker BioSpin as it contains the nose-cone and other apparatus essential for conducting the imaging experiments. A 1/4 inch thick, 82-mm diameter polycarbonate tubing was chosen as the body for the hypobaric chamber with two end caps that are removable (Fig 1A). On one end provisions were made to allow for a 4-channel phased array receiver coil and for providing anesthesia gas. On the other end cap provisions were made to allow for the insertion of a temperature probe, respiration probe, warm water circulation, tubing for vacuum generation, and anesthesia gas circulation (see Fig 1B & C ). Once all the specific items are in place, including the animal, the chamber is hermetically sealed and the pressure in the chamber controlled by adjusting the air flow from a constant vacuum generator, e.g., 12.7 psi (4,000ft altitude, 10.9 psi (8,000 ft altitude) and 4.4 psi (30000-ft altitude). A 86-mm circular-polarized volume is then slipped over the plastic chamber and positioned at the center of the rat head as shown in Fig 1B.Results
The hypobaric chamber was bench tested to assess whether it could maintained a desired hypobaric condition from 12.7 psi, down to 4.4psi. Over several trials (minimum of 4) at each of the above three pressure levels, the chamber was stable and maintained under specific hypobaric condition. Then the chamber was tested inside of the Bruker Biospec 7.0 Tesla 30-cm horizontal bore scanner equipped with a BGA12S gradient system, which was interfaced to a Paravision 6.0 console. Animals were placed prone on an animal bed through 1-3 % isoflurane with 100 % oxygen administration at 1 L/min rate. The head was fixed with a byte bar and a pair of ear pins. The 4-channel RF receiver coil was centered and fixed over the head. The chamber tube was then slid over the animal bed and the two ends of the chamber were sealed with rubber plugs and plastic caps. An MR-compatible system was used to monitor respiration rate and body temperature was maintained at 35-37.5 oC, using a circulating warm water heater. Figure 2a demonstrates high-quality T2-weighted images from the above setup from a female adult Sprague Dawley rat (300 grams) at hypobaric levels of 0.14 x100 kpa (4000 feet). Images were obtained using the RARE sequence at a TR/TE=3000/22 ms, RARE factor=4, slice thickness (st)=1mm, field of view (FOV)=3.5 x 3.5 cm2, in-plane resolution=137 x 137 μm2, average=2. The signal-to-noise of the images from the hypobaric chamber was comparable to those obtained without the use of the chamber. The proton spectra (Fig 2b) obtained from the cortex using a short-TE PRESS (3.5 x 1.5 x 4 mm3), TR/TE=2500/10 ms, and 400 averages clearly demonstrates that the apparatus does not impose additional field inhomogeneity and that excellent quality spectra can be obtained within the chamber. Figure 3, shows fractional anisotropy maps obtained from diffusion tensor images obtained at a TR/TE=2500/24.5 ms, 30 directions, b-value=1000 and 2000 s/mm2, FOV=3.5 x 3.5 cm2, st=1 mm, Matrix size=128 x 128, and 5 bo images to improve the signal to noise of the DTI maps. Clearly the hypobaric chamber does not introduce any additional image distortion.Conclusion
The ability to monitor changes in the brain by simulating an aeromedical environment immediately following brain injury allows one to carefully assess the ramifications of such evacuation on the long term sequelae. In addition other interventions such as normoxic and hyperoxic conditions could be assessed and injury types such as with and without hemorrhagic shock could be assessed while still remaining in the hypobaric condition. We have demonstrated here that such experiments are possible through the use of this hypobaric chamber to study brain changes in vivo.Acknowledgements
We thank the Core for Translational Research in Imaging @ Maryland (C-TRIM) provided in vivo neuroimaging service for the study. We also thank USAMDA medical prototype laboratory to build the hypobaric chamber for the study. This material is based on research sponsored by 711 HPW/XPT under Cooperative Agreement number FA8650-11-2-6142 and FA8650-17-2-6H13. The U.S. Government is authorized to reproduce and distribute reprints for Governmental purposes notwithstanding any copyright notation thereon. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of 711HPW/XPT or the U.S. Government.References
- McGuire JA, Sherman PM, Dean E, Bernot JM, Rowland LM, McGuire SA, Kochunov PV. Utilization of MRI for Cerebral White Matter Injury in a Hypobaric Swine Model-Validation of Technique. Mil Med. 2017 May;182(5):e1757-e1764.
- Proctor JL, Mello KT, Fang R, Puche AC, Rosenthal RE, Fourney WL, Leiste UH, Fiskum G. Aeromedical evacuation-relevant hypobaria worsens axonal and neurologic injury in rats after underbody blast-induced hyperacceleration. J Trauma Acute Care Surg. 2017 Jul;83(1 Suppl 1):S35-S42.
- Skovira JW, Kabadi SV, Wu J, Zhao Z, DuBose J, Rosenthal R, Fiskum G, Faden AI. Simulated Aeromedical Evacuation Exacerbates Experimental Brain Injury.J Neurotrauma. 2016 Jul 15;33(14):1292-302.
Figures
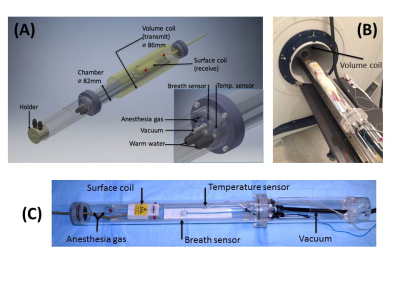
Figure 1. (A) Sketch
of the hyperbaric chamber with surface and volume coils. (B) The chamber with a
live rat (300g) mounted inside the scanner room with inner pressure of 12.7 psi.
(C) The chamber outside the scanner room.
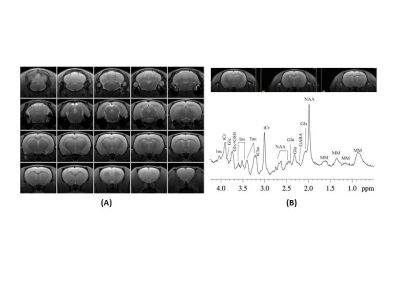
Figure 2. (A)
Structural MRI, RARE, TR/TE=3000/22 ms, RARE factor=4, slice thickness = 1mm,
field of view = 3.5 x 3.5 cm2, in-plane resolution=137 x 137 μm2, averages=2; (B) Short-TE
PRESS on Cortex (3.5 x 1.5 x 4 mm3), TR/TE=2500/10 ms, average=400.
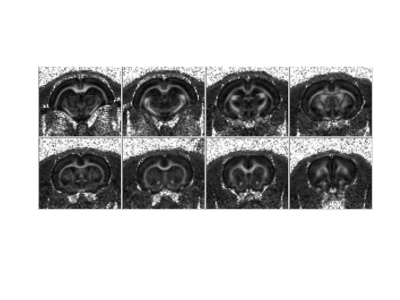
Figure 3.
Fractional anisotropy (FA) maps from diffusion tensor images TR/TE=2500/24.5 ms, 30 directions, b-value = 1000 and 2000 s/mm2, FOV=3.5
x 3.5 cm2, st=1 mm, Matrix size=128 x 128, b0=5