0938
GRAPPA Reconstructed 3D Wave-CAIPI TSE at 7 Tesla1German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany, 2Department of Physics and Astronomy, University of Bonn, Bonn, Germany
Synopsis
In this work, wave-CAIPI sampling is incorporated in a 3D variable flip angle turbo spin echo (TSE) sequence optimized for ultra high field applications. The acquired wave-CAIPI data are reconstructed with a GRAPPA-based wave-CAIPI reconstruction algorithm using multiple reconstruction kernels. Highly accelerated wave-CAIPI TSE images with 1 mm3 resolution and whole brain coverage are acquired at a 7 Tesla scanner. A clear SNR improvement compared to Cartesian CAIPIRINHA is shown.
Introduction
The TSE sequence provides an image contrast that is dominated by the transverse relaxation time T2. It is widely used in clinical applications and neuroscience for T2-weighted brain imaging, structural imaging and lesion detection. Although multiple phase encoding lines are acquired during each repetition time (TR) interval compared to the conventional spin echo sequence, the sequence suffers from long scan times due to the long TR. It has been shown that wave-CAIPI 1 allows highly accelerated 3D imaging with reduced noise enhancement compared to conventional 2D-CAIPIRINHA 2. Wave-CAIPI has been applied to simultaneous multi- slice TSE sequences at a 3 Tesla scanner 3. The aim of this project was to implement a 3D wave-CAIPI TSE sequence optimized for ultra high field applications. The acquisitions were reconstructed using a GRAPPA-based wave-CAIPI image reconstruction 4,5.Methods
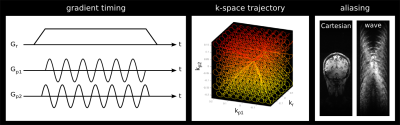
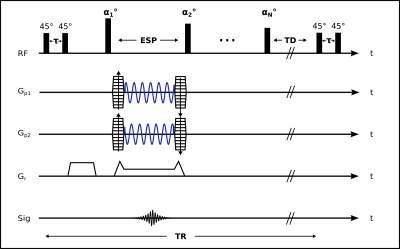
Wave-CAIPI fully exploits the 3D coil sensitivity variations by combining corkscrew k-space trajectories with CAIPIRINHA sampling (see Figure 1). As schematically shown in Figure 2, wave-CAIPI encoding is incorporated in a 3D non-selective variable flip angle TSE sequence 6,7. The flip angles of the sequence are optimized to achieve highest possible SNR at 7T for a predefined signal shape.
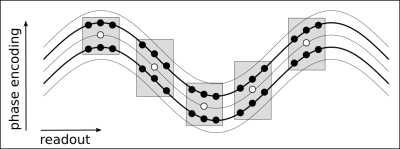
The acquired data were reconstructed using a GRAPPA-based image reconstruction with multiple reconstruction kernel along the readout direction 8 as depicted in Figure 3. After the GRAPPA reconstruction, the image still shows aliasing in readout direction due to the non-Cartesian wave trajectory (Figure 1, right). The remaining aliasing is removed by convolution with a point spread function 1 that depends on the k-space trajectory and the spatial location in the two phase encoding directions. The actual corkscrew k-space trajectory was measured using the Clip-On Camera (Skope, Zurich, Switzerland). It consists of 16 NMR field probes 9 which are placed around the head receive coil and allow to measure the magnetic field dynamics during the MRI acquisition.
All experiments were performed on a MAGNETOM 7T scanner (Siemens Healthineers, Erlangen, Germany) utilizing a 32-channel head coil (Nova Medical, Wilmington, USA). The data were acquired in a healthy volunteer using the following sequence parameters: FOV = 256 x 240 x 176 mm3 (whole brain coverage with readout in head-foot direction), 1 mm3 resolution, TR = 4 s, number of refocusing pulses N = 150 and BW = 300 Hz/pixel. CAIPIRINHA-type accelerated measurements (R=6x4 with shift 2) were performed for Cartesian k-space sampling and wave sampling (16 oscillations/readout and wave amplitudes of A = 2·Δk in both phase encoding directions). Additional scan time reduction was achieved through elliptical sampling. A central dataset of size 512 x 32x 32 served as autocalibration signal (ACS) for the image reconstruction. The total scan time was 1:16 min.
The data were reconstructed using an in-house developed python toolbox for image reconstruction. A 3D reconstruction kernel of size 3 x 3 x 3 was chosen and Tikhonov regularization was used for the calculation of the GRAPPA weights. The ACS lines were included in the GRAPPA reconstruction. G-factor maps were calculated from 100 pseudo multiple replicas 10 and the maximum and mean g-factor was evaluated over the whole brain extracted using BET (FSL 11).
Results
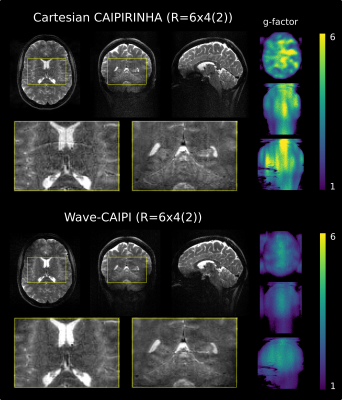
The reconstructed images and g-factor maps of 24-fold accelerated CAIPIRINHA and wave-CAIPI TSE are shown in Figure 4. The wave-CAIPI TSE images show a clear improvement in image quality with less reconstruction artifacts. Noise enhancement is quantified with g-factor maps which is strongly improved using wave sampling. The mean g-factor is reduced 40 % (4.1 without and 2.5 with wave encoding).
Compared to the Cartesian images, the wave-CAIPI images show residual low frequent inhomogeneities (e.g. in the upper left corner of the axial view in Figure 4), which most likely result from improper moment dephasing of the waves. This artifact will be removed in the next steps of our work.
Discussion & Conclusion
We have shown highly accelerated 3D wave-CAIPI TSE images reconstructed with a GRAPPA-based algorithm utilizing multiple reconstruction kernels. 1 mm isotropic whole-brain TSE images were acquired in 1:16 min. Scan time can further be reduced by using separate reference scans with shorter TR. Compared to Cartesian CAIPIRINHA, the wave-CAIPI TSE data achieve improved image quality with less artifacts and strongly reduced g-factor penalties.Acknowledgements
No acknowledgement found.References
1. Bilgic B, Gagoski BA, Cauley SF, Fan AP, Polimeni JR, Grant PE, Wald LL, Setsompop K. Wave-CAIPI for highly accelerated 3D imaging. Magn. Reson. Med. 2015;73(6):2152-2162.
2. Gagoski BA, Bilgic B, Eichner C, Bhat H, Grant PE, Wald LL, Setsompop K. RARE/Turbo Spin Echo Imaging with Simultaneous MultiSlice Wave-CAIPI. Magn. Reson. Med. 2015;73(3):929-938.
3. Breuer FA, Blaimer M, Mueller MF, Seiberlich N, Heidemann RM, Griswold MA, Jakob PM. Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA). Magn. Reson. Med. 2006;55(3):549-556.
4. Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized Autocalibrating Partially Parallel Acquisitions (GRAPPA). Magn. Reson. Med. 2002;47(6):1202-1210.
5. Schwarz MJ, Brenner D, Pracht ED, Stoecker T. GRAPPA Reconstructed Wave-CAIPI MPRAGE at 7 Tesla. Proc. ISMRM 2016.
6. Mugler JP. Optimized three-dimensional fast-spin-echo MRI. J Magn. Reson. Imaging 2014;39(4):745-67.
7. Pracht ED, Feiweier T, Ehses P, Brenner D, Roebroeck A, Weber B, Stoecker T. SAR and Scan-Time Optimized 3D Whole-Brain Double Inversion Recovery Imaging at 7T. Magn. Reson. Med. 2017.
8. Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized Autocalibrating Partially Parallel Acquisitions (GRAPPA). Magn. Reson. Med. 2002;47(6):1202-1210.
9. De Zanche N, Barmet C, Nordmeyer-Massner JA, Pruessmann KP. NMR Probes for measuring magnetic fields and field dynamics in MR systems. Magn. Reson. Med. 2008;60(1):176-186.
10. Robson PM, Grant AK, Madhuranthakam AJ, Lattanzi R, Sodickson DK, Mckenzie CA. Comprehensive Quantification of SNR Ratio and g-Factor for Image-Based and k-space Based Parallel Imaging Reconstructions. Magn. Reson. Med. 2008;60(4):895-907.
11. Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143-155.
Figures