0937
Highly-accelerated volumetric brain protocol using optimized Wave-CAIPI encoding1Department of Physics and Astronomy, Heidelberg University, Heidelberg, Germany, 2Department of Radiology, A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 3Siemens Healthcare GmbH, Erlangen, Germany, 4Harvard Medical School, Boston, MA, United States, 5Harvard-MIT Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States, 6Department of Radiology, Massachusetts General Hospital, Boston, MA, United States
Synopsis
We introduce an optimized six-minute high-resolution whole-brain protocol of 3D, mainly isotropic acquisitions for T2w SPACE, FLAIR (3D), T1w MPRAGE and T2*w SWI; all acquired at 9-fold acceleration using Wave-CAIPI encoding. Design strategies were developed to optimize wave-encoding parameter selection, coil sensitivity computation, and data-driven automated gradient trajectory error estimation. We validated our exam on five volunteers on a 3T MRI scanner and achieved good SNR with negligible g-factor noise and good image contrast despite the 9-fold acceleration. Our fast high-resolution neurological exam should benefit several clinical and research applications and help mitigate patient compliance issues and motion artifacts.
INTRODUCTION
WAVE-CAIPI [1] is a recent parallel imaging technique which combines 2D-CAIPI [2] and Bunch Phase Encoding (BPE) [3] to provide high accelerations with negligible noise amplification. In this work, Wave-CAIPI was employed to create an optimized six-minute high-resolution (mainly isotropic) whole-brain 3D protocol with 9-fold acceleration at 3T. This rapid neurological exam includes prototype sequences for T2w SPACE, FLAIR (3D), T1w MPRAGE, and T2*w SWI, mostly reformattable into any plane without additional reacquisitions. Design strategies were developed to optimize wave-encoding parameters, coil sensitivity computation, and data-driven gradient-trajectory error estimation. This was demonstrated to help achieve robust and high-quality imaging. The resulting fast, high-resolution brain protocol has the potential to improve the speed and efficiency of clinical and research exams (currently 2-4 fold accelerated), and mitigate patient compliance and motion issues.METHODS
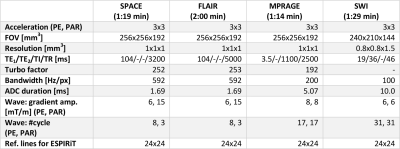
The Wave-CAIPI acquisition scheme was implemented into T2w SPACE, FLAIR (3D), T1w MPRAGE, and T2*w SWI prototype sequences. Auto-calibrated reconstruction [4] was implemented for all sequences to correct for wave-gradient trajectory errors during the image reconstruction.
To ensure robust, high-quality imaging, the following design strategies were investigated:
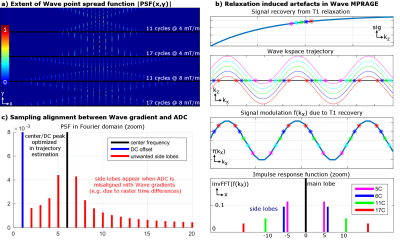
- We analyzed the effect of wave parameters (gradient amplitude & number of sinusoidal cycles per readout) on g-factor noise, and blurring/ringing artifacts caused by signal modulation from T2 relaxation or T1 recovery (see Fig.3a,b).
- In MRI systems, the gradient raster time is typically fixed, while the ADC’s raster time varies depending on the acquisition parameters. We examined artifacts in an auto-calibrated reconstruction when the wave-gradient’s and ADC’s raster time is a non-multiple integer of one another (see Fig.3c).
- The use of short-TE 3D-GRE acquisition and ESPIRiT [5] for coil sensitivity estimation was examined and optimizations of the pre-scan matrix size and ESPIRiT parameters were performed.
In compliance with IRB requirements, five healthy volunteers were scanned on a 3T MRI Scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) using a 32-channel head coil.
Results
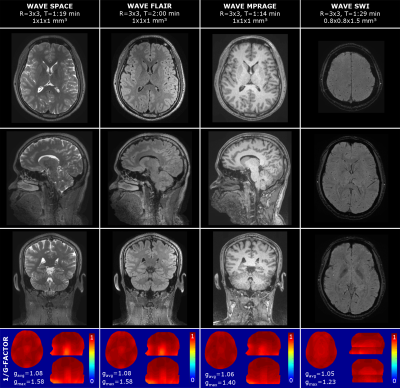
Fig.1 shows results from a six-minute Wave protocol optimized based on findings from Fig.3-5 (see details below). Detailed acquisition parameters are provided in Fig.2. Despite high acceleration (R=3x3), all scans achieve good SNR and contrast, which is reflected in the low g-factor maps (see Fig.1). Here, gavg is close to 1 for all acquisitions, with minor hotspots in the bottom part of the brain. Hotspots are highest for the SPACE sequences (T2w and FLAIR) where larger bandwidth (592 Hz/px) was used in comparison to MPRAGE (200 Hz/px) and SWI (100 Hz/px). This limits the wave-spreading and g-factor performance.
Fig.3a illustrates that the extent of Wave spreading (i.e. controlled-aliasing along x, which correlates to g-factor performance) is mainly determined by the Wave’s gradient amplitude and not the number of wave cycles. However, using a high #cycles will reduce the Wave’s k-space radius for a given amplitude, which results in much less mixing of signal from different partition/phase-encoding steps experiencing different signal modulation due to T1 recovery or T2 decay (e.g. in MPRAGE, SPACE). This in turn leads to a sharp point-spread-function with very small side-lobes (Fig.3b) and reduced artifacts as shown in Fig.4b,c.
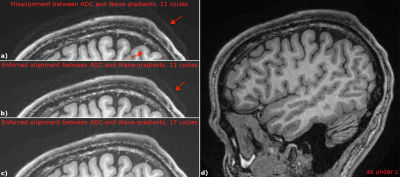
In Fig.3c the wave frequency of the shown PSF does not exactly agree with the ADC duration due to differences in raster times. This removes the sparse nature of the PSF in frequency space as additional side-lobes appear besides the center frequency and DC peak, which are not estimated in the auto-calibrated reconstruction. We mitigated associated artefacts by enforcing the Wave cycle duration to be an exact integer multiple of the ADC duration, as experimentally validated in Fig.4a,b.
We found that ESPIRiT can robustly obtain accurate coil sensitivities using just 24x24 k-space lines of our 3D-GRE. To mitigate potential artifacts from motion during the exam the fast pre-scan (2s) was performed prior to each Wave acquisition.
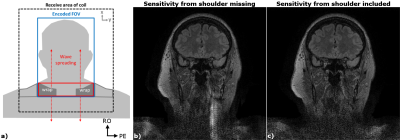
Typically, missing coil sensitivity information from the shoulder causes FOV wraps in the corresponding phase/partition-encoding plane. However, for Wave, such wraps can cause artefacts which spread along the readout (head-food) direction (due to incorrect deblurring) into the area of interest, e.g. the brain (see Fig.5a,b). The use of a second set of sensitivities from ESPIRiT was able to mitigate this issue which has been incorporated into all reconstructions.
DISCUSSION AND CONCLUSION
In this contribution, a six-minute high-resolution volumetric brain protocol was created using Wave-CAIPI encoding. Through careful optimization of the acquisition and reconstruction, good SNR with negligible g-factor noise and good image contrast were achieved among all our volunteer scans despite 9-fold acceleration. Our fast high-resolution neurological exam should benefit a diverse range of clinical and research applications and help mitigate patient compliance issues and motion artifacts.Acknowledgements
This work was supported in part by NIH research grants: R01EB020613, R01EB019437, R24MH106096, P41EB015896, and the shared instrumentation grants: S10RR023401, S10RR019307, S10RR019254, S10RR023043
References
[1] Bilgic B, et al. Wave-CAIPI for Highly Accelerated 3D Imaging. Magnetic resonance in medicine. 2015;73(6):2152-2162.[2] Breuer FA, et al. Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA). Magnetic resonance in medicine. 2006;55(3):549-56.
[3] Moriguchi H, et al. Bunched phase encoding (BPE): a new fast data acquisition method in MRI. Magnetic resonance in medicine. 2006;55(3):633-48.
[4] Cauley SF, et al. Autocalibrated wave-CAIPI reconstruction; Joint optimization of k-space trajectory and parallel imaging reconstruction. Magnetic resonance in medicine. 2017;78(3):1093–1099
[5] Uecker M, et al. ESPIRiT--an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA. Magnetic resonance in medicine. 2014;71(3):990-1001
Figures