0936
Spiral Imaging on a Compact 3T Scanner with High Performance Gradients1Radiology, Mayo Clinic, Rochester, MN, United States
Synopsis
Recently, a low-cryogen, compact 3T MRI system optimized for brain, extremity and infant imaging was developed. The system is equipped with a high slew rate gradient capable of 80 mT/m maximum gradient amplitude and 700 T/m/s slew rate. Due to its reduced imaging volume (26-cm diameter-spherical-volume), the high gradient amplitude and slew rate can be achieved simultaneously on this system with substantially less peripheral nerve stimulation. Here, we investigate the benefit of performing spiral imaging using high gradient performance available on this system. We demonstrated that the high slew-rate can significantly reduce spiral readout time and therefore reduce off-resonance-induced blurring.
Purpose
The specialized MRI platforms optimized for imaging particular organs (e.g., brain) have generated interest due to their advantages compared to the conventional, whole-body MRI, including easier siting and reduced risk of peripheral nerve stimulation (PNS)1-3. Recently, a low-cryogen, compact 3T (C3T) MRI system capable of imaging brains, extremities, and infants was developed1. It has a 26-cm diameter-spherical-volume, and is equipped with a high-performance gradient (80mT/m maximum gradient amplitude and 700T/m/sec slew-rate), in comparison to the 200T/m/s, 50mT/m conventional, 60-cm bore whole-body system like a GE MR750. On the whole-body systems, the PNS concerns usually impose further, stringent limitations on gradient performance. Therefore, the maximal 200T/m/sec slew-rate (SR) is not always available during readout4. However, due to its reduced size, high gradient amplitude and SR can be achieved simultaneously on the C3T with substantially less PNS1. The high SR can particularly benefit acquisitions such as echo-planar-imaging (EPI) by reducing readout time and consequently susceptibility artifacts5. Similarly, spiral acquisitions can benefit from a high SR. Here, we evaluate the readout time reduction for spiral acquisition based on the standard gradient performance available on a GE750 whole-body system, and the high SR on the C3T. Phantom experiments were performed to demonstrate the reduced susceptibility effect using high SR.Methods
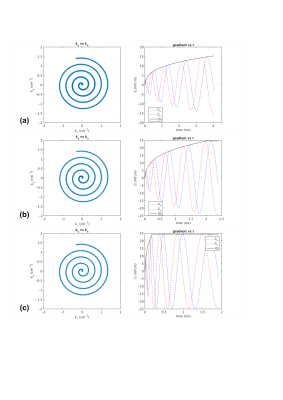
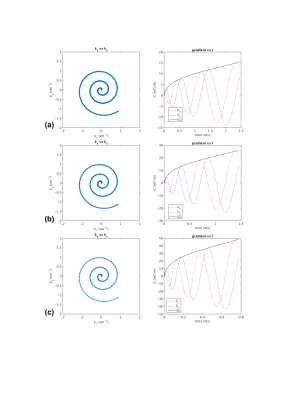
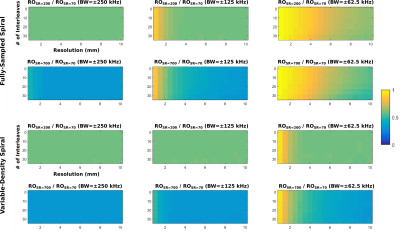
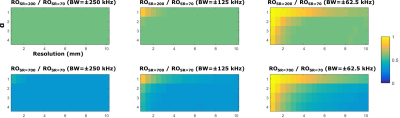
Two-dimensional fully-sampled Archimedean6 and variable-density spiral7 readouts were generated in simulation. On the MR750 system, the SR is by default limited to 70T/m/sec due to PNS risk4. We therefore chose 50mT/s gradient amplitude and 70T/m/sec SR to represent the whole-body system. Simulations were also performed assuming the full gradient SR of the whole-body gradient (200T/m/s) and the C3T (700T/m/s). Simulations were performed assuming a brain scan FOV=24cm, combined with different resolutions (1~10mm), number of interleaves (2~32), and various bandwidth (±250/±125/±62.5kHz). The variable-density spiral was designed using the method described by Lee et al.7. The trajectory is fully-sampled at the k-space center; as the trajectory departs the center, the sampling density gradually decreases in a linear fashion. At each sampling point specified by its k-space radial distance ($$$k_r$$$), the instant sampling rate $$$\Delta k(k_r)=1/FOV-k_r/k_m(1-\alpha)/FOV$$$, i.e., an undersampling factor α is achieved at the end when the maximal radial sampling distance $$$k_m$$$ is reached ($$$\Delta k(k_m)=\alpha/FOV$$$). To demonstrate the advantage of higher SR, the American College of Radiology (ACR) MRI phantom8 was scanned on the C3T using a variable-density spin-echo spiral (FOV=24cm, acquisition resolution=3.5mm, BW=±125kHz, readout=1.4ms, 8 interleaves, α=3, flip angle=90°, 3mm slice, TR/TE=100/7.1ms). The low-resolution setup used here was relevant for applications such as spiral-based arterial spin labeling (ASL)9. The slew rate was set to 200 T/m/s for this initial test. The first/zeroth-order concomitant fields present on the asymmetric gradients were compensated by gradient pre-emphasis10 and frequency tracking11. Images were reconstructed onto a 256×256 matrix using iterative-SENSE solved with conjugate gradient12 without/with off-resonance correction using time segmentation13,14.Results
Figures 1 shows the fully-sampled spiral trajectory and readout gradient designed assuming (a) standard whole-body system SR=70T/m/sec, (b) high SR=200T/m/s, and (c) full SR=700T/m/s on the C3T, all with BW=±125kHz. Figure 2 shows the same plot for a variable-density spiral (α=3). The readout time is reduced from 4.0ms (SR=70) to 2.4ms (SR=200) and 1.9ms (SR=700) for fully-sampled spiral, and from 2.4ms (SR=70) to 1.4ms (SR=200) and 0.8ms (SR=700)) for variable-density spiral. The readout time ratio between the two high SR setups and the standard SR setup was shown in Figure 3 as a function of resolution and number of interleaves for fully-sampled and variable-density spiral (α=3). Figure 4 shows the readout time ratio as a function of undersampling factor α and resolution. A lower number suggests greater readout time reduction. Figure 5 shows an ACR phantom image acquired using variable-density spiral before/after off-resonance correction, as well as the off-resonance map. About 400 rad/s off-resonance was observed at the periphery of the phantom. Due to the short readout time (1.4 ms), off-resonance blurring was much less apparent using a variable-density spiral and high SR.Discussion
These results demonstrate that the high SR available on the C3T system can reduce readout time for spiral acquisitions. Higher SR can especially benefit acquisition typically executed with high BW and moderate-to-low resolution, such as ASL9, fMRI15, and MRE16. Greater readout time reduction is observed when a variable-density spiral is used. The phantom results demonstrate that the high-slew rate can greatly reduce the susceptibility induced blurring. Increasing SR to 700T/m/s can further reduce readout time, but further attention for eddy-current effects may be required17.Conclusion
We demonstrated that the high slew-rate available on the C3T system can reduce spiral readout time and therefore reduce off-resonance-induced blurring.Acknowledgements
This work was supported by NIH grants U01 EB024450-01 and R01 EB010065.References
1. Lee SK, Mathieu JB, Graziani D, et al. Peripheral nerve stimulation characteristics of an asymmetric head-only gradient coil compatible with a high-channel-count receiver array. Magn Reson Med 2016;76:1939–1950.
2. Setsompop K, Kimmlingen R, Eberlein E, et al. Pushing the limits of in vivo diffusion MRI for the Human Connectome Project. NeuroImage 2013;80:220–233.
3. Weiger M, Overweg J, Rösler MB, et al. A high-performance gradient insert for rapid and short-T2 imaging at full duty cycle. Magn Reson Med 2017. DOI: 10.1002/mrm.26954
4. King KF, Schaefer DJ. Spiral scan peripheral nerve stimulation. J Magn Reson Imaging 2010;12:164-170.
5. Tan EK, Lee SK, Weavers PT, etc. High slew-rate head-only gradient for improving distortion in echo planar imaging: Preliminary experience. J Magn Reson Imaging 2016;44:653-664.
6. King KF, Foo TKF, Crawford CR. Optimized gradient waveforms for spiral scanning. Magn Reson Med 1995;34:156-160.
7. Lee JH, Hargreaves BA, Hu BS, Nishimura DG. Fast 3D imaging using variable-density spiral trajectories with applications to limb perfusion. Magn Reson Med 2003;50:1276-85.
8. Phantom test guidance for the ACR MRI accreditation program.Reston: The American College of Radiology, Reston, Virginia, 2005.
9. Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 2015;73:102-116.
10. Tao S, Weavers PT, Trzasko JD, Shu Y, Huston J, Lee S, Frigo LM,Bernstein MA. Gradient pre-emphasis to counteract first-order concomitant fields on asymmetric MRI gradient systems. Magn ResonMed 2017;77:2250–2262.
11. Weavers PT, Tao S, Trzasko JD, Frigo LM, Shu Y, Frick MA, Lee SK, Foo TKF, Bernstein MA. B0 concomitant field compensation for MRI systems employing asymmetric transverse gradient coils. Magn Reson Med 2017. doi:10.1002/mrm.26790.
12. Pruessmann KP, Weiger M, Bornert P, Boesiger P. Advances in sensi-tivity encoding with arbitrary k-space trajectories. Magn Reson Med2001;46:638–651.
13. Noll DC, Meyer CH, Pauly JM, Nishimura DG, Macovski A. A homogeneity correction method for magnetic resonance imaging with time-varying gradients. IEEE Trans Med Imaging 1991;10:629-37.
14. Sutton BP; Noll DC; Fessler JA. Fast, iterative image reconstruction for MRI in the presence of field inhomogeneities. IEEE Trans Med Imaging 2003;22:178-188.
15. Glover GH. Spiral imaging in fMRI. Neuroimage 2012;15:706-12.
16. Johnson CL, McGarry MDJ, van Houten EEW, et al. Magnetic Resonance Elastography of the brain using multi-shot spiral readouts with self-navigated motion correction. Magn Reson Med 2013:70:404-412.
17. Addy NO, Wu HH, Nishimura DG. A simple method for MR gradient system characterization and k-space trajectory estimation. Magn Reson Med 2012;68:120-129.
Figures