0932
Myelin digestion during multiple sclerosis lesion formation contributes to increase on QSMKofi Deh1, Gerald Ponath2, Zaki Molvi1, Gian-Carlo Toriano Parel1, Kelly M. Gillen 1, Shun Zhang1, Thanh Nguyen 1, Pascal Spincemaille1, Yinghua Ma 1, Ajay Gupta 1, Susan Gauthier 1, David Pitt2, and Yi Wang1
1Weill Cornell Medicine, New York, NY, United States, 2Yale School of Medicine, New Haven, CT, United States
Synopsis
During the initial processes in the formation of MS lesions, myelin is immediately digested in macrophages after phacocytosis. Chemical bond breakdown in myelin basic proteins (MBP) and among lipid bilayers (LB) can increase magnetic susceptibility. Here we measure susceptibility increase in phantom experiments of MBP breakdown and myelin LB breakdown. We investigate myelin degradation in the first few weeks of MS lesion formation by performing histology on MS brain samples and by in vivo imaging of enhancing lesions in MS patients.
Purpose
To assess destruction of myelin in new MS lesions and corresponding increase in lesion magnetic susceptibility.Theory
Loss of chemical bonds in biomolecules leads to decreased diamagnetism because the formerly bonding electrons become more tightly held by parent nuclei leading to a reduced “induced” magnetic field following the application of a magnetic field. This decrease can be estimated using Pascal’s rule which states that the magnetic susceptibility of a compound is the sum of the magnetic susceptibilities of the constituent atoms (or appropriately chosen subunits) and bonds [1]. When a peptide bond is broken during proteolysis of the myelin basic protein (MBP), the resulting change in volume susceptibility can be estimated to be 37.1 ppb [2]. Human trypsin cleaves MBP at 3 sites to give a total change of 111 ppb [3]. Pascal’s rule implies that the volume susceptibility of a mixture will increase when bonds are broken between components of a mixture. The increase is proportional to the free energy change associated with the reaction, which is about 4.9 kJ/mol for a membrane protein [4], or 14 ppb for extracting MBP from the myelin lipid bilayer [5].Methods
Actively demyelinating white matter lesions were stained with Oil Red O (ORO) and fluoromyelin lipid stains. Confocal imaging was performed on lesions stained with the macrophage marker, CD68, and the nuclear stain Hoechst 33342. Bovine myelin basic protein (MBP, Sigma-Aldrich, U.S.) in PBS was incubated with trypsin in a 37°C water bath for 2 hours, with controls, consisting of pure PBS, MBP in PBS, and trypsin in PBS. Aliquots were subjected to immunoblotting using an antibody to MBP (Santa Cruz Biotechnology) and embedded in a 1% agarose gel phantom for MR imaging with a 3D gradient-echo sequence. Purified human myelin was digested with 13% sodium dodecyl sulfate (SDS) for 16 hours at 37°C. Myelin breakdown was verified using fluoromyelin and Bodipy fluorescent stains. GRE imaging was performed on phantoms of digested myelin and controls. QSM maps of the phantoms were reconstructed using MEDI+0 QSM reconstruction [6]. The volume susceptibility of the digested samples and controls were measured by taking the mean voxel value of a spherical region of interest (ROI) placed in the region occupied by the sample within the agarose gel medium. The susceptibility change due to sample digestion was recorded. Three relapsing-remitting MS patients were scanned with standard T1 and T2 weighted imaging for anatomy, multi-echo GRE imaging for QSM, and gadolinium-enhanced imaging at recruitment and within three weeks of the initial scan as part of an ongoing longitudinal human MRI study. Brain QSM maps were reconstructed using MEDI+0.Results
ORO lipid staining of an acutely demyelinating MS lesion showed punctuate staining indicative of broken down myelin. Staining with the with the macrophage marker CD68, fluoromyelin, and the nuclear stain Hoechst 33342 showed the presence of myelin fragments inside phagocytosing macrophages (Fig. 1). ROI-based estimates from QSM maps showed that digestion of myelin by trypsin caused volume susceptibility to increase by an average of 112 ± 37 ppb in close agreement with the theoretical estimate of 111 ppb (Fig. 2). Degradation of purified myelin with SDS resulted in a substantial reduction of fluoromyelin intensity (Fig.3c and d). Bodipy staining of myelin before and after SDS treatment confirmed lipid breakdown into smaller liposomes (Fig 3e and f). ROI-based estimates from QSM maps showed myelin degradation caused volume susceptibility to increase by 23 ppb. In three patients with new Gd enhancing lesions, lesion susceptibility values above the contralateral NAWM were 12.9, 9.9 and 1.1 ppb. Figure 4 shows an example of the susceptibility increase during the formation of a new MS lesion which was initially hyperintense on a Gd-enhanced T1 weighted image (Fig. 4a) and isointense on QSM (Fig. 4c). After 19 days, this lesion was no longer Gd-enhancing (Fig. 4b) and became slightly hyperintense on QSM (Fig. 3d) as indicated by the susceptibility increase of 9.9 ppb.Discussion
In-vitro QSM volume susceptibility change of 112 ± 37 ppb, close to a theoretical estimate of 111 ppb, for MBP digestion, and 23 ppb for myelin denaturation. Myelin and proteins are estimated to constitute 14% and 4% of white matter [7], respectively, giving total susceptibility change due to protein digestion and myelin denaturation to be (4% × 112 ppb) + (14% + 23 ppb), which is approximately 7.8 ppb, close to the average estimate of 8 ppb obtained in vivo. Myelin digestion in the initial days contributes to susceptibility increase, while is consistent with reported MRI phase change.Acknowledgements
This work was supported in part by grants from the National Institutes of Health (R01 NS090464, S10 OD021782) and the National Multiple Sclerosis Society (RG-1602-07671).References
[1]Pascal, ACP, 9(19):5,1910, [2] Babaei, JCTC, 13, 2945, 2017, [3] Medveczky, FEBS Letters 580 (2006) 545, [4] Martenson, Myelin, 1992, [5] Takahashi, 1994;67(11):2967, [6] Liu, MRM, 78, 303, 2017, [7] Quarles, Chapter 4, Basic NeurochemistryFigures

Figure 1. Acutely demyelinating white matter lesion. (A) staining with the lipid
dye, Oil Red O (ORO), demonstrates punctate staining within the lesion
(magnification of rectangle 1 in B). ORO+ lipids are indicative
of broken-down myelin. (C) Confocal image of the lesion stained with the
macrophage marker CD68 (green), fluoromyelin (red) and nuclear stain Hoechst
33342 (blue) demonstrates the presence of myelin fragments within phagocytosing
macrophages. (D) Magnification of rectangle 2 shows intact ORO+ myelin
in the normal appearing white matter.
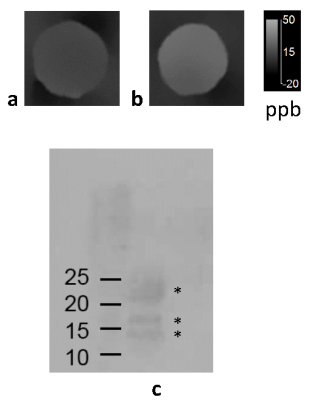
Figure
2. QSM
of myelin basic protein (a) and MBP digested for 2 hours with trypsin (b)
embedded in a gel phantom. Western blotting of MBP (c; left lane) and MBP
incubated for two hours with trypsin (c; right lane), confirm the digestion of
MBP by trypsin. The first lane shows three isoforms of MBP (marked by
asterisks), with apparent molecular weights of 14, 17 and 21 kDa, respectively.
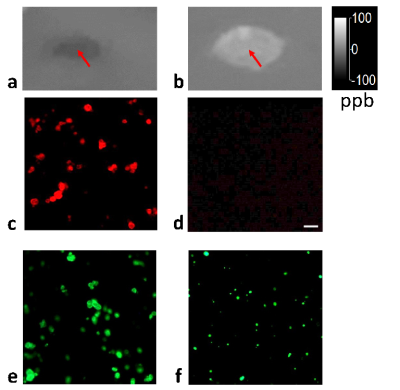
Figure
3. QSM
of homogenized purified human myelin (arrow) embedded in a gel phantom (a) and
myelin degraded by sodium dodecyl sulfate (SDS). Corresponding lipid stains
show that lipids in intact myelin are visible when stained with fluoromyelin (c) and Bodipy 493/503 (e), while
lipids released from myelin treated with SDS are no longer visible with
fluoromyelin (d) but still visible as smaller fluorescent dots with the Bodipy
stain (f).
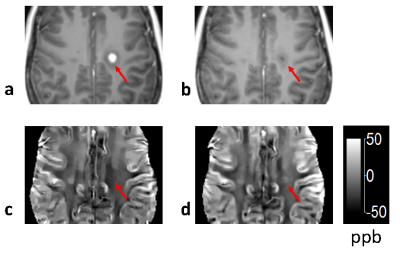
Figure 4. Gd
enhanced T1 weighted images (a and b) and corresponding QSM (c and d) of a new
acute MS lesion acquired at the time of Gd enhancing and 19 days later. The BBB
is closed as evidenced by the non-enhancing appearance in (b). Notice the
increase in susceptibility within the lesion (arrow), suggesting myelin
digestion by macrophages.