0929
Neuroplastic Changes of Myelin Microstructure With Video Game Play1Waisman Center, University of Wisconsin Madison, Madison, WI, United States, 2Medical Physics, University of Wisconsin Madison, Madison, WI, United States, 3Psychology, University of Wisconsin Madison, Madison, WI, United States, 4Psychiatry, University of Wisconsin Madison, Madison, WI, United States
Synopsis
Mounting evidence suggests that changes in the brain can occur within hours, however, the mechanisms underlying these changes remain unknown. In this work, we utilized multicomponent relaxometry (mcDESPOT) to examine the effects of both short and long term video game playing on myelinated white matter. Short and long term changes in quantitative longitudinal relaxation times as well as long term changes of myelin water fraction were observed. These results add to the growing literature that neuroplastic effects can take place over the short term while also suggesting long term changes may involve mechanisms of myelination.
Introduction
The ability of the brain to reorganize, a phenomenon known as neuroplasticity, is not only essential to our capacity to acquire new knowledge and skills but also to compensate for injury. Studies utilizing magnetic resonance imaging (MRI) have begun to detect and characterize the underlying short and long-term morphometric, microstructural, and functional changes associated with repetitive trainings1-3. A recent study using video games (racecar game) observed measurable decreases in DTI mean diffusivity (MD) in the hippocampus and parahippocampus following a period of 90 minutes of play4. In a similar study, decreased MD was again detected in the hippocampus and posterior-dorsal dendate gyrus, while increases in functional connectivity and behavioral performance were also observed after 45 minutes of play5. However, while these studies importantly demonstrate the ability to detect neuroplastic changes in the brain, the underlying mechanisms of these neuroplastic changes remain unknown. In regard to microstructural changes, changes in DTI are inherently non-specific and may be influenced by local astrocyte swelling, changes in synapses and dendrites, or even changes in myelin6. Alternative imaging techniques, such as relaxometry based methods, may provide more insight into the underlying microstructural changes. In this work, we examine both short and longer term effects of video game play on the myelin microstructure using multicomponent driven equilibrium single pulse observation of T1 and T2 (mcDESPOT)7.Methods
MRI Acquisition: A cohort of 20 subjects (6 Males, 14 Females) were enrolled as part of a larger study examining the effects of video game training on neuroplasticity and took part in a similar spatial learning and memory training as previously examined4. MRI scanning was performed at three separate occasions on a GE MR750 3T scanner and using a 32-channel head RF coil: 1) prior to any training, 2) after 90 minutes of training, and 3) after completing 15 hours of training spread over 6 weeks and 10 training sessions. During the training sessions, participants played the Need for Speed video game and completed 16 trials of the same track in the same vehicle. Between each trial, participants ordered images of the track and attempted to draw the course from memory in a drawing tool within MATLAB. mcDESPOT imaging, which consists of multiple flip angle SPGR and bSSFP images (two-phase cycles), were acquired during each MRI scan for a total of 3 datasets for each individual. Actual flip angle imaging (AFI) was additionally collected for correction of B1-field inhomogeneity. Data subsequently underwent three-pool mcDESPOT processing8 and parameter maps of the myelin water fraction (VFM), longitudinal (T1) and transverse (T2) relaxation times were calculated. Advanced Normalization Tools (ANTs)9 was used to create a study specific template, align this study specific template to the MNI template, and bring parameter maps (i.e. VFM, T1, T2) into the MNI space. Statistical Analysis: Analyses consisted of comparing baseline VFM, T1, and T2 measurements to measurements after training was performed. Paired t-tests were performed using non-parametric permutation testing with FSL’s randomise tool10, 10000 permutations and threshold-free cluster enhancement for correction of multiple comparisons11.Results
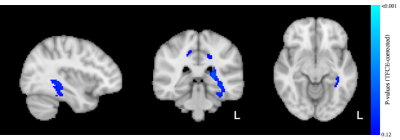
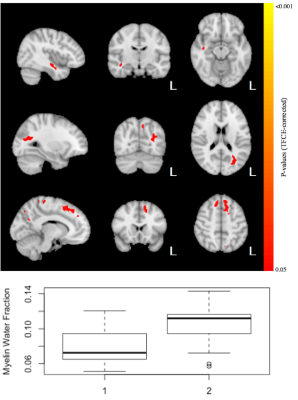
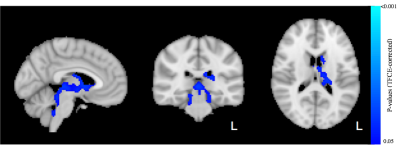
A total of 14 subjects had usable mcDESPOT data at all 3 testing occasions. No significant differences after 90 minutes of play were observed for VFM or T2. However, a trend relationship (p<0.12) of decreased T1 after 90 minutes of video game training was detected (Fig 1). These relationships were observed to span regions of left hemisphere temporal lobe and cerebral white matter, including the thalamic radiations, parahippocampal gyrus, hippocampus, and temporal fusiform cortex. Longer term changes (after 6 weeks of training) in VFM and T1 were also detected. In particular, increases (p<0.05) of VFM in the left inferior fronto-occipital fasciculus, superior frontal white matter, and right inferior longitudinal fasciculus were observed (Fig. 2). Decreased in T1was observed in sensory motor areas, including the thalamus, thalamic radiations, brain stem, and caudate (Fig 3). No changes in T2 were observed.Conclusion
Our results indicate that relaxometry based methods may additionally be sensitive to neuroplastic changes in the brain and therefore may provide additional insight into the underlying mechanisms involved. In particular, longer-term increases of VFM and decreases of T1 are consistent with a hypothesis of learning inducing changes in myelination, while short term changes in T1, may indicate alterations of local water content. Future analyses will begin to compare this cohort to a group that underwent a different video game training paradigm as well as a training-naïve group.Acknowledgements
This work was supported in part by the National Institutes of Mental (K99MH110596, DCD, PI). Infrastructure support was also provided, in part, by a core grant to the Waisman Center from the National Institute of Child Health and Human Development (U54 HD090256).References
1. Sampaio-Baptista, C, Khrapitchev, AA, Foxley, S, Schlagheck, T, Scholz, J, Jbabdi, S, DeLuca, GC, Miller, KL, Taylor, A, Thomas, N, Kleim, J, Sibson, NR, Bannerman, D, Johansen-Berg, H, 2013. Motor skill learning induces changes in white matter microstructure and myelination. J Neurosci 33, 19499-19503.
2. Taubert, M, Draganski, B, Anwander, A, Muller, K, Horstmann, A, Villringer, A, Ragert, P, 2010. Dynamic properties of human brain structure: learning-related changes in cortical areas and associated fiber connections. J Neurosci 30, 11670-11677.
3. Taubert, M, Lohmann, G, Margulies, DS, Villringer, A, Ragert, P, 2011. Long-term effects of motor training on resting-state networks and underlying brain structure. NeuroImage 57, 1492-1498.
4. Sagi Y, Tavor I, Hofstetter S, Tzur-Moryosef S, Blumenfeld-Katzir T, Assaf Y. Learning in the Fast Lane: New Insights into Neuroplasticity. Neuron 73:1195–1203
5. Keller TA, Just MA. Structural and functional neuroplasticity in human learning of spatial routes. Neuroimage 2016;125:256–266.
6. Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don'ts of diffusion MRI. NeuroImage 73:239–254
7. Deoni SC, Rutt BK, Arun T, Pierpaoli C, Jones DK. Gleaning multicomponent T1 and T2 information from steady-state imaging data. Magnetic Resonance in Medicine 60:1372–1387
8. Deoni SC, Matthews L, Kolind SH. One component? Two components? Three? The effect of including a nonexchanging “free” water component in multicomponent driven equilibrium single pulse observation of T1 and T2. Magnetic Resonance in Medicine 70:147–154
9. Avants B, Epstein C, Grossman M, Gee J. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Medical image analysis 12:26–41.
10. Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. NeuroImage 92:381–397
11. Smith S, Nichols T. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44:83–98
Figures