0909
Systemic Inflammation Adversely Affects Mitochondrial Function as Assessed by 31P MRS of Thigh Muscle with Exercise1Laboratory of Clinical Investigation, National Institute on Aging, National Institutes of Health, Baltimore, MD, United States, 2Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, GA, United States, 3Longitudinal Studies Section, National Institute on Aging, National Institutes of Health, Baltimore, MD, United States
Synopsis
Systemic inflammation has been shown to play a role in both the aging process and disease progression. However, the effect of inflammation on mitochondrial function has not been fully elucidated. We hypothesized that increased systemic inflammation, assessed through measurement of conventional markers of inflammation, would be accompanied by a decrease in mitochondrial function, as assessed by 31P MRS of leg muscle. Our results suggest that increased systemic inflammation may adversely affect mitochondrial function.
PURPOSE
Systemic
inflammation, resulting in the production of pro-inflammatory markers including
interleukin-6 (IL-6) and C-reactive protein (CRP) as well as increased erythrocyte
sedimentation rate (ESR), represents a homeostatic response to insult.1
However, inappropriate inflammation can result in a range of pathologies. Indeed, increased inflammation is correlated
with physical frailty in the aging population2 and with risk of morbidity
and mortality. Impaired mitochondrial
function is an additional correlate with increasing age, with 31P magnetic
resonance spectroscopy (MRS) being used extensively to assess mitochondrial
function in vivo3. Mitochondrial function has been found to be
impaired in the settings of, for example, cancer 4 and
neurodegenerative diseases.5 Conversely, increased mitochondrial
function is correlated with improved muscle function, such as gait speed and muscle
strength, in the aging population.6 While the roles of mitochondrial function and
inflammation in the aging process have been independently investigated, the
association between mitochondrial function and systemic inflammation has not
been well established. We sought to elucidate this relationship using
conventional markers of inflammation and a metric of mitochondrial function, the
rate of PCr recovery following exercise (kPCr), obtained from 31P
MRS of lower extremity muscle, in a large sample of normatively aging subjects.
EXPERIMENTAL DESIGN & METHODS
530 subjects from the Baltimore Longitudinal Study on Aging (BLSA) were studied: 241 males (mean age 70.75; range 24-91) and 289 females (mean age 69.92; age 24-98). Characteristics of the study population, including mean values of outcome measures, are shown in Table 1. ESR, CRP concentration, and IL-6 concentration were measured using standard laboratory techniques. 31P spectra of leg muscle were acquired using a 3T Achieva MR scanner (Philips, Best, The Netherlands). Subjects were supine, and a 10-cm 31P-tuned surface coil (PulseTeq, Surrey, UK) was fastened over the vastus lateralis muscle of the left thigh. A single-pulse sequence was employed with a 90° adiabatic excitation pulse followed by acquisition of 2048 points with a bandwidth of 2250 Hz, repetition time 1.5 s and four averages, resulting in a time resolution of 6 s. After collection of 9 resting spectra, subjects performed a knee extension exercise until the PCr peak height fell to 33% to 66% of its baseline value. Additional spectra were acquired during recovery, so that a total of 75 spectra were collected over 7.5 min. Spectra were processed using jMRUI (version 5.0).7,8 The recovery rate of phosphocreatine, kPCr =1/τPCr, was used as a metric of muscle oxidative phosphorylation capacity. kPCr was determined by fitting the postexercise recovery of PCr to a mono-exponential function:
$$PCr(t) = PCr_{0} + ΔPCr\ (1- exp(-t/τ_{PCr}))$$
where PCr0 is PCr peak area after completion of exercise, ΔPCr is the reduction in PCr peak area from baseline at end-exercise, and τPCr is the PCr exponential recovery time constant.6 We ensured accurate quantification of kPCr through application of specific quality control criteria. Independent linear regression analyses were performed using RStudio to determine the relationship between kPCr and each inflammatory marker, with quantitative results obtained after adjusting for age, sex, race, BMI and degree of PCr depletion.
RESULTS & DISCUSSION
Phosphocreatine recovery rate (kPCr) was evaluated as a function of ESR (Fig. 1), CRP concentration (Fig. 2) and IL-6 concentration (Fig. 3), with a statistically significant inverse relationship being found for all three markers. These relationships persisted after adjustment for age as a confounding variable. The effect size was relatively small, indicating that, as expected, other factors also play a role in decreased kPCr. However, the significant relationship between systemic inflammation is of great significance, since inflammation is a known correlate with the progression of aging and disease. For example, higher CRP levels are correlated with decreased muscle mass, independent of age and body composition,9 with similar findings observed for IL-6.10 In addition, systemic inflammation is negatively correlated with muscle function in specific pathologies; IL-6 is negatively correlated with exercise capacity and muscle strength in elderly subjects who have obstructive lung disease (OLD) as well as subjects without this diagnosis.11 In addition, sarcopenic and sarcopenic-obese (SO) patients present with increased systemic inflammation that correlates with functional limitations.12 Thus, systemic inflammation may play a central role in both normative and non-normative aging, and the correlation between inflammation and muscle bioenergetics provides a plausible basis for the attendant functional limitations.
CONCLUSIONS
ESR, CRP concentration, and IL-6 concentration are significantly associated with a decrease in muscle oxidative capacity as assessed by 31P MRS. These results, obtained on a normative population of healthy aging individuals, motivate additional investigation of the relationship between muscle bioenergetics and systemic inflammation in the setting of specific pathologies, especially those for which inflammation is a critical component.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institute on Aging of the National Institutes of Health.
References
1. Krishnamoorthy, S. and K.V. Honn, Inflammation and disease progression. Cancer Metastasis Rev, 2006. 25(3): p. 481-91.
2. Tay, L., et al., The Independent Role of Inflammation in Physical Frailty among Older Adults with Mild Cognitive Impairment and Mild-to-Moderate Alzheimer's Disease. J Nutr Health Aging, 2016. 20(3): p. 288-99.
3. Lanza, I.R., et al., Measurement of human skeletal muscle oxidative capacity by 31P-MR spectroscopy: a cross-validation with in vitro measurements. J Magn Reson Imaging, 2011. 34(5): p. 1143-50.
4. Boland, M.L., A.H. Chourasia, and K.F. Macleod, Mitochondrial dysfunction in cancer. Front Oncol, 2013. 3: p. 292.
5. Johri, A. and M.F. Beal, Mitochondrial dysfunction in neurodegenerative diseases. J Pharmacol Exp Ther, 2012. 342(3): p. 619-30.
6. Zane, A.C., et al., Muscle strength mediates the relationship between mitochondrial energetics and walking performance. Aging Cell, 2017. 16(3): p. 461-468.
7. Vanhamme, L., et al., Time-domain quantification of series of biomedical magnetic resonance spectroscopy signals. J Magn Reson, 1999. 140(1): p. 120-30.
8. Naressi, A., et al., Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med, 2001. 31(4): p. 269-86.
9. Atkins, J.L., et al., Low muscle mass in older men: the role of lifestyle, diet and cardiovascular risk factors. J Nutr Health Aging, 2014. 18(1): p. 26-33.
10. Schaap, L.A., et al., Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med, 2006. 119(6): p. 526 e9-17.
11. Yende, S., et al., Inflammatory markers are associated with ventilatory limitation and muscle dysfunction in obstructive lung disease in well functioning elderly subjects. Thorax, 2006. 61(1): p. 10-16.
12. Levine, M.E. and E.M. Crimmins, The impact of insulin resistance and inflammation on the association between sarcopenic obesity and physical functioning. Obesity (Silver Spring), 2012. 20(10): p. 2101-6.
Figures
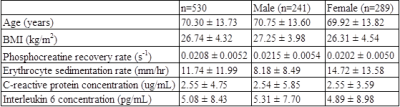
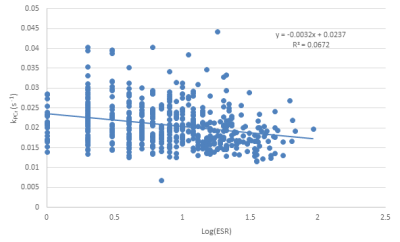
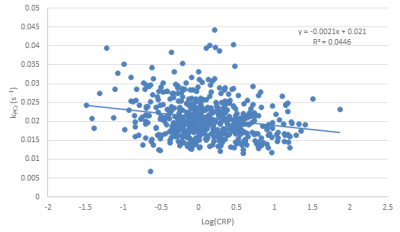
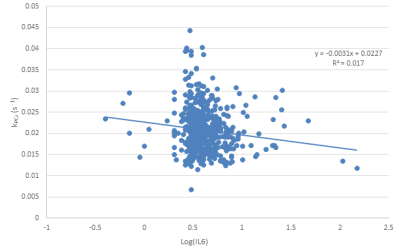
Figure 3: Correlation between log-transformed interleukin 6 (IL-6) concentration obtained from blood samples and phosphocreatine recovery rate obtained from 31P MRS. Note the inverse correlation (p=0.05), indicating decreased mitochondrial function associated with increased IL-6 concentration. Importantly, this relationship remained significant after adjustment for age.