0907
A Pilot Study for In Vivo Measurement and Quantification of Brain Glucose Metabolic Rates using Oral Uptake of Deuterated GlucoseMing Lu1, Xiao-Hong Zhu1, Yi Zhang1, and Wei Chen1
1Center for Magnetic Resonance Research, Department of Radiology, University of Minnesota, Minneapolis, MN, United States
Synopsis
Recently, we developed a novel Deuterium MRS (DMRS) approach for simultaneously measuring cerebral glucose consumption rate and TCA cycle flux in rat brains at 16.4 T. Instead of using a clamp protocol in 13C MRS studies, our DMRS approach utilizes a brief i.v. infusion of glucose isotope. In this work, we aimed to establish a completely noninvasive delivery of the tracer into brain. By using oral uptake, DMRS detection sensitivity was evaluated and metabolic rates were quantified. Our results demonstrated the feasibility of using oral uptake for DMRS applications, which makes it highly suitable and promising for translation to patients.
Introduction
Quantification and imaging of cerebral glucose consumption rate (CMRglc) and tricarboxylic acid cycle flux (VTCA) is crucial for understanding neuroenergetics. Recently, we developed a novel Deuterium (2H) MRS (DMRS) approach for simultaneously measuring CMRglc and VTCA in rat brains at ultrahigh field (1). This new method has the advantages of eliminating the usage of radioactive tracer employed by 18FDG-PET imaging for CMRglc measurement; and of allowing more signal averaging resulting in substantial sensitivity gains due to the much shorter T1 of deuterated glucose (~0.05 s), when comparing with 13C MRS for VTCA quantification (1, 2). Instead of using a clamp protocol for 13C MRS studies to keep constant plasma 13C-glucose level during hours of measurement, the DMRS approach utilizes a brief i.v. infusion of deuterated glucose as a substance and tracer (1). To further simplify the protocol, in this work, we aimed to establish a completely noninvasive delivery of the glucose isotope into the brain. By using oral uptake, DMRS detection sensitivity was evaluated and the metabolic rates were quantified and compared with those measured through i.v. infusion in the rat brain.Method
Sprague Dawley rats were anesthetized by 2% isoflurane. The femoral arteries were catheterized for blood sampling and physiological monitoring. For the rat under infusion protocol, femoral veins were also catheterized for deuterated glucose injection. For the rat using oral uptake, commercial standard chow was replaced by 25% sugar water for overnight. In the morning of experimental day, a feeding tube was inserted from the mouth, through the throat and esophagus into the stomach for delivery of tracer before animal entering magnet. All MR scans were conducted at 16.4 T/26 cm scanner (Varian/VNMRJ) using 1H/2H surface coil. A single-pulse-acquire sequence was applied to obtain dynamic 2H spectra from the rat brain (1). For each rat, 5~10 min baseline spectra were acquired followed by 2 min i.v. infusion of 1.3 g/kg D-Glucose-6,6-d2 (d66, Sigma-Aldrich) or a bolus injection of 1.9 g/kg d66 through the feeding tube. Metabolites concentrations including glucose (Glc) and glutamate/glutamine (Glx) were quantified as previously described (1). Exponential (for i.v. infusion) or polynomial (for oral uptake) functions were used to describe the changes of blood glucose level, which served as inputs of a kinetic model (1) for determination of CMRglc and VTCA.Result
Figure 1 showed excellent DMR spectral quality with four well-resolved deuteriated signals (water, Glc, Glx and lactate (Lac)) detected in the rat brain after oral uptake of d66. As shown in Fig. 2A, the dynamics of blood glucose level under the oral uptake protocol was significantly different from that of using i.v. infusion. Although the dose of d66 used for oral uptake was higher (~46%), the peak of blood glucose level appeared later (delayed by ~30 min) and lower (~half) when delivering the tracer into the stomach instead of directly into the veins. This was due to the dilution effects of the orally taken glucose by other tissues/organs (such as liver) prior to entering into arteries, which resulted in a constantly lower blood glucose level during the whole experiment when comparing to i.v. infusion. Similarly, because of this slower and down-regulated blood glucose behavior as an input to the brain, time courses of cerebral Glc and Glx concentrations under oral uptake protocol displayed an altered dynamics (Fig. 2B vs. 2C). As shown in Fig. 2B, excellent model fittings provided reliable estimations of metabolic rates: 0.2 μmol/g/min for CMRglc and 0.6 μmol/g/min for VTCA, which were consistent with the values obtained from the brain using i.v. infusion at the same anesthesia level (1).Discussion & Conclusion
The results indicate that in vivo DMRS approach is robust and reliable for simultaneously assessing CMRglc and VTCA using different delivery methods for the glucose isotope, i.e., oral uptake or i.v. infusion. While high dose was applied for oral uptake to compensate the dilution effects from other tissues/organs, the efficiency of deuterated glucose utilization by the brain needs to be improved to further increase the DMRS detection sensitivity. For example, employing somatostatin would be a choice, which inhibits insulin and glucagon secretion to reduce the conversion between the labeled glucose and glycogen in liver (3). In summary, this pilot study demonstrated the feasibility of using oral uptake of deuterated glucose for DMRS to quantitatively determine the metabolic rates in vivo in a completely noninvasive way, which makes this technique highly suitable and promising for translation to patients.Acknowledgements
NIH Grants: R01 NS41262, NS57560, NS70839, MH111447, R24 MH106049, P41 EB015894, P30 NS076408, S10 RR025031 and Keck foundation.References
(1) Lu, M. et al. (2017) J Creb Blood Flow Metab., PMID: 28503999. (2) Gruetter, R. et al. (1994) J. Neurochem. 63, 1377-1385. (3) Chen, W. et al. (2001) Magn Reson Med. 45, 349-355.Figures
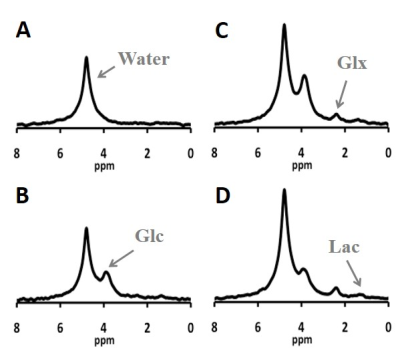
Figure 1. DMR spectra obtained from a representative rat brain under 2% isoflurane
before (A), 20 min (B), 60 min (C) and 90 min (D) after oral uptake of
deuterated glucose. Each spectrum was summed from 1 min of data acquisitions (4
spectra).
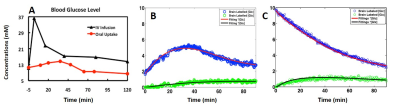
Figure 2. Time courses of blood glucose concentrations (A) and the model-fitting
results obtained from two representative rat brains under 2% isoflurane
anesthesia using oral uptake (B) or i.v. infusion (C) of deuterated glucose at
the 0th min.