0900
Early Prediction of Language Deficits in Very Preterm Infants Using Functional Connectome Data and Machine Learning1Perinatal Institute, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, United States, 2Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH, United States, 3Pediatric Neuroimaging Research Consortium, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, United States
Synopsis
Children who are born prematurely are at an increased risk for impaired neurodevelopmental outcomes, including language deficits. Earlier identification, soon after birth, of infants who are experiencing difficulties with complex language function is urgently needed to take advantage of critical windows of brain development so that targeted delivery of Early Intervention therapies can be undertaken during this optimal period. We propose to develop a robust machine learning framework that can analyze functional brain connectome data obtained at term corrected age to make an individual-level prediction about language outcomes at two years corrected age in very preterm infants.
INTRODUCTION
Children who are born prematurely are at increased risk for neurodevelopmental impairments,1 including language deficits/difficulites.2 Earlier identification, soon after birth, of infants who are experiencing difficulties with complex language function, is urgently needed to take advantage of critical windows of brain development so that targeted delivery of Early Intervention therapies can be undertaken during this optimal period. Prelinguistic communication, which are typically through actions and behaviors without using words, begins to develop immediately after birth. 3 It has been recognized that different combinations of risk and protective factors may contribute to predict the outcomes of language deficits/difficulties for the child born preterm. 4-6 Atypical brain connectivity has also been reported in other populations that exhibit language deficits.7 In this work, we propose to develop a robust machine learning framework that can analyze functional brain connectome data obtained at term corrected age to make an individual-level prediction about language outcomes at two years corrected age in very preterm infants.METHODS
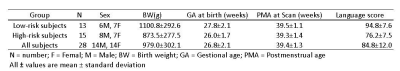
The study population was derived from a prospective cohort of very preterm infants (<32 weeks gestational age) from the NICU at Nationwide Children’s Hospital. Imaging specifications were: 3T GE HDx scanner with 8-channel infant head coil. T2w – TR/TE 11000/185 ms, FA 90°, resolution 0.35 x 0.35 x 2 mm; functional connectivity MRI (fcMRI) – EPI TR/TE 3000/35 ms, FA 90°, resolution 2.8 x 2.8 x 3 mm. Standardized Bayley-III test was conducted at 2 years corrected age. The Bayley-III language scores are on a scale of 50 to 150, with a mean of 100 and standard deviation of 15. We grouped our cohort using a cut-off of 86 in those at high vs. low risk for language deficits (i.e. two classes). The demographic information for these infants are provided in Table 1. fcMRI data were preprocessed using our established neonatal-optimized pipeline. 8 We then analyzed resting state functional connectivity using functional connectivity toolbox (CONN; MIT, Cambridge, US). Ninety region of interests (ROIs) were defined based on a neonatal automated anatomical labeling (AAL) atlas. 9 The edges in the functional connectome describe the degree of functional connectivity; defined as a partial correlation between two ROIs. This resulted in a 90 x 90 adjacency matrix symmetric about the diagonal, in which each entry represented the brain connectivity between each pair of ROIs. We implemented Stacked Sparse Autoencoder (SSAE) to take functional connectome as input and extract its high-level connectome features (these features capture the embedded salient information that is useful for differentiating a single subject). We then implemented support vector machine (SVM) to conduct 2-class classification (i.e. high risk vs. low risk) using extracted functional brain connectome features. The proposed machine learning model was unsupervised and trained using brain connectome data from ~1600 synthetic subjects 10 and cross-validated on 28 very preterm infants. We compared the prediction performance of SVM among several approaches, including using raw functional connectome features (noted as Raw + SVM), high-level connectome features extracted via principle components analysis (PCA; with top 10 components, noted as PCA + SVM) and via our proposed SSAE approach (noted as SSAE + SVM). The performance of the classification is assessed using conventional metrics of accuracy, specificity and sensitivity and area under the receiver operating characteristic curve (AUC).RESULTS
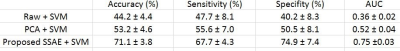
As shown in Table 2, the prediction using raw connectome features performed as expected – worse than random guessing on a two-classification problem. This poor prediction was caused by a “curse of dimensionality” problem, because the feature size was far greater than the sample size. To overcome this problem, we proposed to use SSAE to reduce the dimensionality of functional connectome features and compared this with conventional PCA approach. Using SSAE to extract high-level brain connectome features, we accurately classified 71.1% of very preterm infants at high risk of language deficits with 67.7% sensitivity, 74.9% specificity and 0.75 AUC. The improvements as compared with PCA in accuracy, sensitivity, specificity and AUC were 17.9%, 12.1%, 24.4% and 0.23, respectively.DISCUSSIONS AND CONCLUSIONS
In this study, we proposed a machine learning framework to predict language deficits, soon after birth, in very preterm infants using brain connectome data. Our study shows that functional brain connectome data are useful as prognostic biomarkers when employing SSAE. The prediction accuracy is expected to be further improved by incorporating clinical risk factors, for example, gestational age, gender, brain injury, hearing status, socio-economic risk factors and environment. This work shows a proof of concept for using machine learning on connectome data to capture the individual variability inherent in the developing brain of preterm neonates. A larger study is important to validate our approach.Acknowledgements
Funded in part by NIH grant R01 NS094200-02 from the National Institutes of Neurological Diseases and Stroke (NAP).
References
1. Glass HC, Stayer SA, Brett C, et al., Outcomes for extremely premature infants. Anesth Analg, 2015; 120(6): 1337–1351.
2. van Noort-van der Spek IL, Franken MC, Weisglas-Kuperus N, Language functions in preterm-born children: a systematic review and meta-analysis. Pediatrics, 2012; 29: 745-754.
3. Rossetti LM (Ed.), Communication intervention: birth to three, 2nd ed., 2001, Clifton Park, NY: Delmar.
4. Loeb DM, Predictors of Language Outcome for Children Born Preterm: Implications for Early Intervention. Communication Disorders, Deaf Studies & Hearing Aids, 2014; 2:e114.
5. Grunau RV, Kearney SM, Whitfield MF. Language development at 3 years in pre-term children of birth weight below 1000 g. Br J Disord Commun 1990;25:173e82.
6. Marston L, Peacock JL, Calvert SA, et al., Factors affecting vocabulary acquisition at age 2 in children born between 23 and 28 weeks’ gestation. Dev Med Child Neurol 2007;49:591e6.
7. Verly M, Verhoeven J, Zink I, et al., Altered functional connectivity of the language network in ASD: Role of classical language areas and cerebellum, Neuroimage Clin. 2014; 4: 374–382.
8. He L, Parikh NA. Aberrant Executive and Frontoparietal Functional Connectivity in Very Preterm Infants With Diffuse White Matter Abnormalities. Pediatr Neurol, 2015; 53(4), 330-337.
9. Shi F., Yap PT, Wu G., et al., Infant brain atlases from neonates to 1- and 2-year-olds. PLoS One, 2011. 6(4): p. e18746.
10.He H, Bai Y, Garcia EA, et al., ADASYN: Adaptive synthetic sampling approach for imbalanced learning. Paper presented at: IEEE International Joint conference on Neural Networks. 2008; Hong Kong.