0898
Identifying Individual Motor Function Using Machine Learning Predication Based on Resting-State fMRI for Presurgical Mapping in Patients with Brain TumorChen Niu1, Elizabeth Zakszewski2, Alexander Cohen2, Xiao Ling1, Ming Zhang1, Maode Wang1, and Yang Wang2
1First Affiliated Hospital of Xi'An Jiaotong University, Shaanxi Xi'an, China, 2Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
A novel machine learning model was developed using resting-state and task fMRI on healthy subjects. This study applied this novel model to clinical patients. Preliminary data on 25 patients with space-occupying brain tumors suggested our approach could accurately predict hand functional area at the individual level in patients with brain tumors, even in cases where patients had displacement of brain tissue and reorganization of brain motor functional network. Our methods implicated the great potential for clinical application of presurgical mapping.
Introduction
Resting-state functional MRI (rs-fMRI) has provided new insights on the functional architecture of the healthy brain. Because it is noninvasive and does not require patient cooperation, rs-fMRI may be particularly useful in clinical patients. However, preoperative fMRI is performed exclusively in individual patients and therefore differs fundamentally from research applications in the neurosciences. Most recently, a novel model based on machine learning (ML) was developed using Human Connectome Project data to predict individual differences in brain activity.1 This model highlights a coupling between brain connectivity and function that can be captured at the individual subject level.1,2 Here, we applied this novel ML model to patients with space-occupying brain tumors, to predict hand movement functional area from clinical rs-fMRI data alone, using activation maps of both active and passive motor task fMRI from the same patients as a reference for comparison.Subjects and Methods
Twenty-five patients with brain tumors (M/F=12/13, age 51.3±15.8 years) were studied on a 3T MR scanner. For each patient, both structural imaging (3D SPGR 1x1x1 mm3) and fMRI scans (including resting state and task) using the BOLD EPI sequence (TR/TE = 2500/30ms, FA=90, voxel size=3.75x3.75x3mm3, 132 imaging frames) were acquired. To evaluate the predicted hand motor functional map generated from rs-fMRI, two types of block-designed hand movement tasks (active and passive hand movement) were conducted on all patients. For the active hand movement task, patients were asked to repetitively open and close their bilateral hands at a steady frequency following the visually presented clues, and to remain still during the rest period. During the passive hand movement task, patients were asked to relax while the examiner repetitively opened and closed one of their hands at the same frequency as during the active movement task. Each hand was examined separately using the passive movement task. All fMRI data were preprocessed using the Human Connectome Project (HCP) minimal preprocessing pipeline3 and converted to the CIFTI format, where data were normalized into standard space using ANTs (Advanced Normalization Tools).4,5 As described in detail elsewhere,1,2 a ML model was built and “trained” based on rs-fMRI and hand movement task fMRI data from a group of healthy subjects acquired using the same protocol. The ML model was then applied to patients’ rs-fMRI data to generate a predicted hand motor functional map for each patient. Additionally, individual task activation maps were derived from a general linear model analysis on all task fMRI datasets.Results
Overall, the novel ML model successfully predicted hand motor activation in individual patients. Interestingly, three general patterns were observed: 1) In cases where displacement of the primary hand motor area was not present, the ML-predicted motor activation maps matched very well with both active and passive task activation maps (Fig. 1). 2) In cases where severe gyri compression was present, the ML-predicted maps showed displacement of the activated areas in the affected hemisphere with respect to the contralateral ones, matching activation maps from both active and passive tasks (Fig. 2). 3) In cases where patients showed decreased or no activation of the active task on the affected side, the ML-predicted map still demonstrated that the hand motor region matched well with the activation map of the passive task of the contralateral hand.Discussion and Conclusion
Location of the functional region for hand movement in the primary sensory-motor cortex plays an important role in presurgical planning and risk assessment in patients with brain tumors. fMRI can help detect the motor functional region in the cortex where normal anatomical patterns are lost. Increasing work has applied rs-fMRI to presurgical mapping in patients with possible compliance problems with the task fMRI. However, existing data have shown that rs-fMRI using independent component analysis or the seed-based method failed to determine hand motor functional regions as detected by the hand movement task fMRI in patients with brain tumors.6,7 This study applied a ML model generated using healthy subjects to patients with brain tumors and successfully predicted individual finger motor region, even in cases where patients had displacement of brain tissue and reorganization of brain functional networks. Although rs-fMRI has not yet reached the status of an established and standardized diagnostic neuroimaging procedure, our work indicates a novel approach for presurgical mapping, even without needing to acquire task-based fMRI training data in patients.Acknowledgements
This work was supported by a Daniel M. Soref Charitable Trust Grant (to Y.W.).References
1. Tavor I, Parker-Jones O, Mars RB, et al. Task-free MRI predicts individual differences in brain activity during task performance. Science. 2016;352(6282):216-220. 2. Parker Jones O, Voets NL, Adcock AE, et al. Resting connectivity predicts task activation in pre-surgical patients. Neuroimage Clin. 2017;13:378-385. 3. Glasser MF, Sotiropoulos SN, Wilson JA, et al. WU-Minn HCP Consortium. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105-24. 4. Tustison NJ, Cook PA, Klein A, et al. Large scale evaluation of ANTs and FreeSurfer cortical thickness measurements. Neuroimage. 2014; 99:166-179. 5. Tustison NJ, Shrinidhi KL, Wintermark M, et al. Optimal symmetric multimodal templates and concatenated random forests for supervised brain tumor segmentation (Simplified) with ANTsR. Neuroinformatics. 2015;13(2):209-225. 6. Hou BL, Bhatia S, Carpenter JS. Quantitative comparisons on hand motor functional areas determined by resting state and task BOLD fMRI and anatomical MRI for pre-surgical planning of patients with brain tumors. Neuroimage Clin. 2016;11:378-387. 7. Zhang M, Niu C, Lin P, et al. Resting-state fMRI for presurgical motor cortex mapping is not enough. ISMRM 2011;2126.Figures
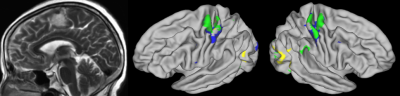
Fig.
1.
Case 19. F, 60y, arachnoid fibroblastoma located at left supplementary motor
area. Bilateral finger motor activation predicted from rs-fMRI matches well
with activation generated from finger movement task fMRI. (blue: predicted
activation; yellow: task activation; green: overlap)
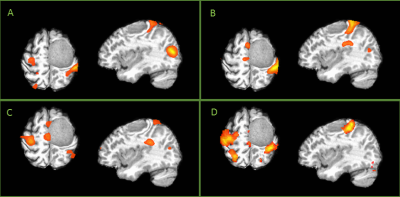
Fig.
2.
Case 18. M, 54y, left frontal arachnoid fibroblastoma. A) Activation of active
bilateral finger movement task fMRI; B) Activation of passive right hand
movement task fMRI; C) Activation of passive left hand movement task fMRI; D)
Predicated activation map derived from rs-fMRI.