0872
Absolute quantification of resting oxygen metabolism and cerebral physiology following acute exposure to repeated sub-concussive collisions: A calibrated MRI approach1Centre of Neuroscience studies at Queen's University, Kingston, ON, Canada, 2Cardiff University Brain Research Imaging Centre, Cardiff, United Kingdom, 3Department of Surgery at Queen's University, Kingston, ON, Canada
Synopsis
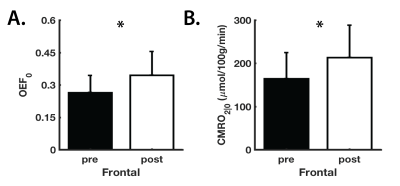
Athletes involved in collision sports can sustain up to ~1400 head impacts, within a single season. In this study, we explore the physiological nature of sub-concussive impacts using a calibrated MRI method, which combines hypercapnic and hyperoxic breathing manipulations. Collegiate football players were scanned at pre-season baseline and following three weeks of practices and one game. Relative to baseline, we observed global increased resting oxygen extraction fraction (OEF0) and cerebral metabolic rate of oxygen consumption (CMRO2|0) in the grey-matter, with significant differences found within the frontal lobe (P<0.05). These results emphasize the need to regulate exposure to head impacts in sports.
Introduction
In recent years, there has been a growing concern that exposure to repeated sub-concussive head impacts may lead to long-term changes in cognitive functions.1–3 Despite not triggering symptoms, recent evidence also suggests that season-long exposure to sub-concussive trauma can cause transient changes in brain physiology, similar to those that occur immediately after a sport-related concussion.4,5 Thus, longitudinal exposure to such impacts may play a cumulative role in the uncoupling between cerebral blood flow (CBF) and metabolic rate of oxygen consumption (CMRO2), which acts as a substrate for the neuropathological cascade that follows a concussion.6 In this study, we implement the calibrated MRI method proposed by Bulte et al. (2012)7 to evaluate the acute effects of repeated sub-concussive head trauma on regional hemodynamic parameters. We hypothesized that increases in metabolic demands will be observed acutely following cumulative exposure to repeated sub-concussive head trauma, relative to baseline, as a reflection of increased resting oxidative metabolism.4,8Methods
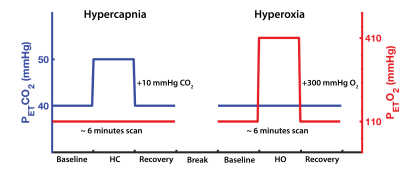
A cohort of sixteen collegiate football student-athletes (N=16; meanage = 20 ± 1 years) underwent functional imaging on a Siemens 3.0T scanner (using a 32-channel receiver head coil) at pre-season baseline (pre-), prior to any participation in contact practices, and following three weeks of exposure to sub-concussive collisions during fall training camp (post-). For each MRI session, subjects completed two 6 minutes boxcar respiratory breathing challenges (one hypercapnia, and one hyperoxia; Figure 1), induced using a feed-forward computerized gas delivery system (RespirActTM, Thornhill Research Inc., Toronto, ON).
BOLD and CBF data were acquired simultaneously using a dual-echo pseudo-continuous arterial spin labeling sequence (pCASL), and parameters inspired from Alsop et al. (2015)9: TR = 4000 ms, TE1/TE2 = 10/30 ms, field of view = 250 x 250 mm, flip angle = 90°, voxel size = 3.9 mm isotropic, post-labeling delay (PLD) = 1000 ms, tagging duration 1.665s.10 Perfusion weighted images for each block design were extracted from the first echo (TE = 10 ms) using a linear surround subtraction between the control and tag images,11 and converted to physiological units (mL/100g/min) using the single-blood compartment model.12 Individual BOLD timeseries and CBF images from each breathing manipulations were then computed into the Bulte’s calibrated method7 to estimate the following hemodynamic parameter maps: M (the theoretical maximal percent BOLD change from complete removal of deoxyhemoglobin), resting oxygen extraction fraction (OEF0) and resting cerebral metabolic rate of oxygen consumption (CMRO2|0), while iso-metabolic state during hypercapnia was assumed.13
Following registration into standard MNI space,14 regional parameters between the pre- and post- scans were compared across the grey-matter (GM) using a repeated-measures analysis of variance (R-ANOVA; SPSS Inc., Chicago, IL, USA), and a statistically significant alpha threshold of 0.05.
Results and discussion
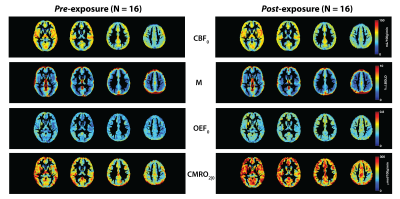
We compared CBF0, M, OEF0 and CMRO2|0 between the pre- and post-exposure sessions within the frontal, parietal, temporal and occipital GM, along with the deep GM, which included the thalamus, caudate, insula and putamen altogether. At baseline (pre-scan), our average values for CBF0 (GMCBF0 = 74±11 mL/100g/min) were higher than ‘normative’ values reported in the current literature.15 This difference (~deltaCBF0 = +10-25 mL/100g/min; Figure 2 in Lajoie et al. (2016)) may be attributed to the fact that our cohort is made of highly fit collegiate athletes, given that CBF velocity, and cerebrovascular health, have been shown to increase with aerobic fitness in healthy men.16 Based on the expected inverse relationship between CBF0 and OEF0,11 higher CBF0 may also contribute to the slightly lower averaged OEF0 values found in the football players at pre-season baseline (OEF0 = 0.28±0.08), when compared to the literature (~deltaOEF0 = -0.01; Figure 2 in Lajoie et al. (2106)). Group averaged GM CMRO2|0 (CMRO2|0 = 182±57 umol/100g/min) was also elevated when compared to the same literature (~deltaCMRO2|0 = +50 umol/100g/min), which is likely attributable to the higher CBF0, given the direct relationship between the two variables.
When compared to pre-season baseline, regional CBF0 and M values showed no significant differences (P>0.05) following acute exposure to sub-concussive collisions (Figure 2). On the other hand, OEF0 and CMRO2|0 showed global increases between the pre- and post-time points, with statistically significant differences (P<0.05) observed within the frontal lobe (Figure 3). Because CBF0 did not change between pre- and post-exposure, we attribute changes in CMRO2|0 to changes in OEF0. These preliminary findings suggest that metabolic demands may be increased following exposure to sub-concussive collisions, which further provides evidence that exposure to such impacts should be regulated across sports, to improve player safety.
Acknowledgements
The authors would like to thank Mr. Don Brien for his contribution to this project and his help with data collection.References
1. Guskiewicz, K. M. et al. Recurrent concussion and risk of depression in retired professional football players. Med. Sci. Sports Exerc. 39, 903–909 (2007).
2. Omalu, B. I., Bailes, J., Hammers, J. L. & Fitzsimmons, R. P. Chronic Traumatic Encephalopathy , Suicides and Parasuicides in. 31, 130–132 (2010).
3. Hampshire, A., MacDonald, A. & Owen, A. M. Hypoconnectivity and hyperfrontality in retired American football players. Sci. Rep. 3, 2972 (2013).
4. Slobounov, S. M. et al. The effect of repetitive subconcussive collisions on brain integrity in collegiate football players over a single football season: A multi-modal neuroimaging study. NeuroImage Clin. 14, 708–718 (2017).
5. Johnson, B., Neuberger, T., Gay, M., Hallett, M. & Slobounov, S. Effects of Subconcussive Head Trauma on the Default Mode Network of the Brain. J. Neurotrauma 31, 1907–1913 (2014).
6. Giza, C. C. & Hovda, D. a. The Neurometabolic Cascade of Concussion. J. Athl. Train. 36, 228–235 (2001).
7. Bulte, D. P. et al. Quantitative measurement of cerebral physiology using respiratory-calibrated MRI. Neuroimage 60, 582–591 (2012).
8. Svaldi, D. O. et al. Cerebrovascular reactivity alterations in asymptomatic high school football players. Dev. Neuropsychol. 40, 80–84 (2015).
9. Alsop, D. C. et al. Recommended implementation of arterial spin-labeled Perfusion mri for clinical applications: A consensus of the ISMRM Perfusion Study group and the European consortium for ASL in dementia. Magn. Reson. Med. 73, 102–116 (2015).
10. Wu, W., Buxton, R. B. & Wong, E. C. Vascular space occupancy weighted imaging with control of residual blood signal and higher contrast-to-noise ratio. IEEE Trans. Med. Imaging 26, 1319–27 (2007).
11. Germuska, M. et al. A forward modelling approach for the estimation of oxygen extraction fraction by calibrated fMRI. Neuroimage 139, 313–323 (2016).
12. Wang, J. et al. Arterial transit time imaging with flow encoding arterial spin tagging (FEAST). Magn. Reson. Med. 50, 599–607 (2003).
13. Hoge, R., Atkinson, J., Gill, B., Crelier, G. & Marrett, S. Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: The …. Magn. Reson. Med. 863, 849–863 (1999).
14. Jenkinson, M., Beckmann, C. F., Behrens, T. E. J., Woolrich, M. W. & Smith, S. M. Fsl. Neuroimage 62, 782–790 (2012).
15. Lajoie, I., Tancredi, F. B. & Hoge, R. D. Regional reproducibility of BOLD calibration parameter M, OEF and resting-state CMRO2 measurements with QUO2 MRI. PLoS One 11, (2016).
16. Ainslie, P. N. et al. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J. Physiol. 586, 4005–4010 (2008).
Figures