0871
Local neuronal synchronization and global functional disconnection are signatures of propofol-induced unconsciousness1University of Michigan, Ann Arbor, MI, United States, 2Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
Information processing in the brain occurs through a hierarchy of temporal receptive windows (TRWs). Anesthetic drugs induce a reversible suppression of consciousness and thus offer a unique opportunity to investigate the state-dependence of TRWs. Here we demonstrate that sedation with propofol is accompanied by the prolongation of the brain’s intrinsic functional timescales, i.e. enlarged TRWs. This is accomplished by an increase of local and regional signal synchronization, which in turn disrupts information exchange among distant brain regions. Finally, we show that the brain’s information processing timescales exhibit distinct dynamic signatures in sedation, deep anesthesia, and disorders of consciousness.
INTRODUCTION
Environmental events are processed on multiple
timescales via hierarchical organization of temporal receptive windows (TRWs) in
the brain [1-5]. The hierarchy of TRWs permits the brain to link multiple perceptual timescales, thus
constructing a temporal continuity of conscious experience [6]. The dependence of TRWs on altered states of consciousness is unclear. Furthermore,
reduced consciousness is marked by a shift toward low-frequency oscillations
and slowing cortical dynamics. We hypothesize that the slowing of neural
dynamics is related to a prolongation of the brain’s intrinsic functional
timescales, i.e., enlarged TRWs, which may also be linked to simultaneous
changes in local and global neuronal interactions that normally support
information integration necessary for consciousness [7,8]. METHODS
To test our hypothesis and potentially underlying mechanisms, we examined the relationship between the brain’s intrinsic timescales (local/voxel level) and its regional (across neighboring voxels) and global (whole brain level) functional connectivity in experiments performed with resting-state fMRI (3T Signa GE 750; TR/TE=2000/25ms) in healthy volunteers undergoing graded levels of sedation with propofol (Dataset-1; n=23; male/female 14/9). Subjects received 15-20 min scans in wakefulness, propofol-induced light (0.98 ± 0.18 μg/ml; OAAS score 4) and deep sedation (1.88 ± 0.24 μg/ml; OAAS score 4), transition and recovery [9,10]. The anesthetic agent propofol was manually administered as guided by computer simulation for target-controlled continuous infusion (STANPUMP) [11] based on the pharmacokinetic model [12]. After standard fMRI preprocessing, we measured the timescales of spontaneous activity using first-order temporal autocorrelation coefficient: AC1=corr(yt, yt-1) where y is the fMRI-BOLD time-course. Higher AC1 indicates a shift toward a longer timescale of slower dynamics. We also measured the fraction of low-frequency standard deviation (fSD), the SD ratio of band-passed BOLD signals at 0.02-0.06 Hz and 0.06-0.1Hz, which was previously shown to reflect the size of TRWs [1,2,5]. Regional homogeneity (ReHo) [13], global connectivity – averaged weighted degree of centrality (wDC) [14], and topographical similarity (Topo) [15] across different stages of propofol sedation were assessed and compared with the TRW indices (AC1 and fSD). Finally, in order to interpret these experiments in relation to other unconscious states, we extended our analysis to data from participants exposed to surgical levels of general anesthesia (Dataset-2; n=12; male/female: 5/7) and patients with disorders of consciousness (DOC) (Dataset-3; n=21; male/female: 14/7).RESULTS
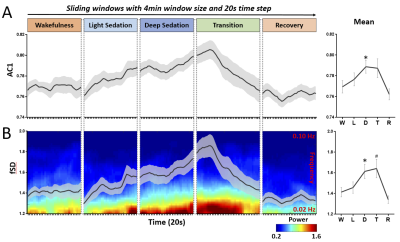
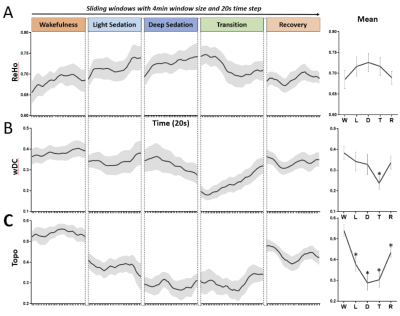
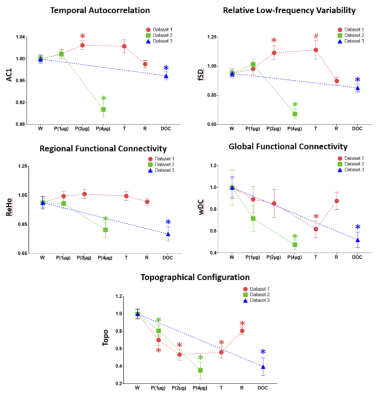
We observed a prolongation of the brain’s intrinsic functional timescales during propofol sedation with an increase of temporal autocorrelation (AC1) and low-frequency variability (fSD) of intrinsic brain activity (Fig. 1). The increase of AC1 and fSD was concomitant with an elevated regional BOLD signal correlation (ReHo), and a breakdown of global functional connectivity (wDC) strength as well as a departure from the presumably optimized spatial configuration of the wakeful baseline (Topo) (Fig. 2). Finally, we showed that deep general anesthesia and disorders of consciousness could be distinguished from the sedated state, by the opposite direction of changes of AC1, fSD and ReHo (Fig. 3).DISCUSSION
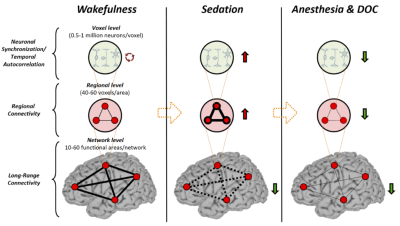
The global increase of AC1 and fSD observed during propofol sedation suggest a prolongation of the brain’s intrinsic functional timescales with an enlarged TRW. The enlarged TRWs may act as low-pass filters or sparse sampling of the inputs from extrinsic and intrinsic sources, reducing the bandwidth of information processing. The increase of AC1 may also indicate an increase of local neuronal synchronization. This was seen at the intermediate spatial scale through increases in the regional homogeneity (ReHo) across neighboring voxels during sedation. In contrast to the increase of local and regional signal correlation, the decrease of global long-range functional connectivity and topographical similarity during sedation may suggest that both the strength and spatial configuration of functional connectivity diverges from that of baseline over time. Given that the TRW indices (AC1 and fSD) first increase during sedation (Dataset-1) and then decrease during deep anesthesia (Dataset-2), we speculate that this biphasic phenomenon results from two consecutive stages of functional alterations. First, an increase of local/regional synchrony breaks down global connectivity during light to moderate sedation; and second, both local/regional synchrony and global connectivity collapse at a high, surgical dose (Fig. 4).CONCLUSION
We demonstrate for the first time that sedation with propofol synchronizes local neuronal interactions and prolongs the intrinsic functional timescales with an enlarged TRW. This, in turn, disrupts information exchange among distant brain regions. The functional processing timescales have distinct neural dynamic signatures in sedation, deep general anesthesia and disorders of consciousness. These results improve our understanding of the neural mechanisms of unconsciousness in pharmacologic and neuropathologic states.Acknowledgements
This study was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award R01-GM103894 and by the Department of Anesthesiology, University of Michigan.References
1. Hasson, U., Yang, E., Vallines, I., Heeger, D. J. & Rubin, N. A hierarchy of temporal receptive windows in human cortex. J. Neurosci. 28, 2539–50 (2008).
2. Honey, C. J. et al. Slow cortical dynamics and the accumulation of information over long timescales. Neuron 76, 423–434 (2012).
3. Lerner, Y., Honey, C. J., Silbert, L. J. & Hasson, U. Topographic mapping of a hierarchy of temporal receptive windows using a narrated story. J. Neurosci. 31, 2906–15 (2011).
4. Murray, J. D. et al. A hierarchy of intrinsic timescales across primate cortex. Nat. Neurosci. 17, 1661–3 (2014).
5. Stephens, G. J. et al. A place for time: the spatiotemporal structure of neural dynamics during natural audition. J. Neurophysiol. 110, 2019–26 (2013).
6. Northoff, G. & Huang, Z. How do the brain’s time and space mediate consciousness and its different dimensions? Temporo-spatial theory of consciousness (TTC). Neurosci. Biobehav. Rev. 80, 630–645 (2017).
7. Alkire, M. T., Hudetz, A. G. & Tononi, G. Consciousness and anesthesia. Science 322, 876–80 (2008).
8. Tononi, G., Boly, M., Massimini, M. & Koch, C. Integrated information theory: from consciousness to its physical substrate. Nat. Rev. Neurosci. 17, 450–61 (2016).
9. Liu, X. et al. Fine-grained parcellation of brain connectivity improves differentiation of states of consciousness during graded propofol sedation. Brain Connect. 7, 373–381 (2017).
10. Liu, X. et al. Propofol attenuates low-frequency fluctuations of resting-state fMRI BOLD signal in the anterior frontal cortex upon loss of consciousness. Neuroimage 147, 295–301 (2017).
11. Shafer, S. STANPUMP User’s Manual. (1996).
12. Marsh, B., Morton, N. & Kenny, G. N. C. Pharmacokinetic model driven infusion of propofol in children. Br. J. Anaesth. 67, 41–48 (1991).
13. Zang, Y., Jiang, T., Lu, Y., He, Y. & Tian, L. Regional homogeneity approach to fMRI data analysis. Neuroimage 22, 394–400 (2004).
14. Zuo, X. N. et al. Network centrality in the human functional connectome. Cereb. Cortex 22, 1862–1875 (2012).
15. Tagliazucchi, E., Chialvo, D. R., Siniatchkin, M., Brichant, J. & Laureys, S. Large-scale signatures of unconsciousness are consistent with a departure from critical dynamics. J. R. Soc. Interface 13, 1–34 (2016).
Figures