0862
Characteristics of Plaques and Lenticulostriate Arteries in Stroke Patients by Whole-Brain Vessel Wall Magnetic Resonance Imaging1Department of Radiology, Xuanwu Hospital, Capital Medical University, Beijing, China, 2Biomedical Imaging Research Institute, Cedars Sinai Medical Center, Los Angeles, CA, United States, 3MR Collaboration NEA, Siemens Healthcare, Beijing, China
Synopsis
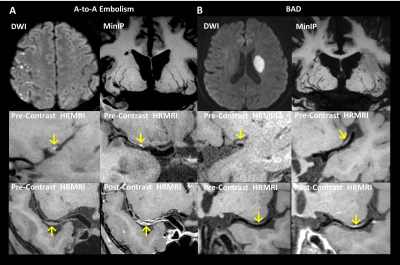
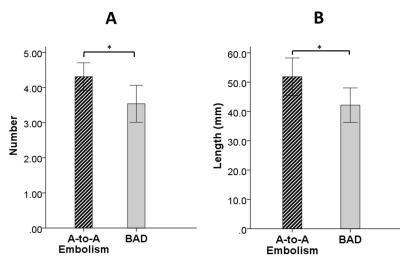
The aim of our study was to investigate the plaque characteristics and the lenticulostriate artery (LSA) status in artery-to-artery (A-to-A) embolism and branch atheromatous disease (BAD) using whole-brain high-resolution MRI. The results showed that patients with A-to-A embolism presented more frequently hyperintense plaques (HIP), plaque surface irregularity, and severe stenosis than patients with BAD. A significant reduction in the number and lengths of LSA branches in patients with BAD compared to A-to-A embolism was also found. Multivariate analysis further demonstrated that HIP was independently associated with A-to-A embolism, whereas the reduction in LSA branches was independently associated with BAD.
Purpose
The mechanisms of intracranial atherosclerotic disease (ICAD) causing stroke are multi-factorial and can likely be inferred from plaque characteristics. 1-3 Nevertheless, few studies have focused on unraveling the difference in plaque features among stroke subtypes, which may potentially aid in accurately diagnosing these strokes. 4,5 Recent studies have demonstrated the feasibility of whole-brain high-resolution magnetic resonance imaging (WB-HRMRI) in visualizing the vessel wall and lenticulostriate arteries (LSA) in one image setting. 6,7 The aim of the present study was to evaluate atherosclerotic plaque characteristics and the LSA using a novel WB-HRMRI method and to establish their relationship with different infarction patterns.Material and Methods
Recent stroke patients with artery-to-artery (A-to-A) embolism or branch atheromatous disease (BAD) identified by DWI were prospectively enrolled and underwent a WB-HRMRI exam. WB-HRMRI was performed on a MAGNETOM Verio 3T MR system (Siemens Healthcare, Erlangen, Germany) using a 3D T1-weighted whole-brain vessel wall MRI prototype technique known as inversion-recovery (IR)-prepared SPACE with the following parameters: TR/TE = 900/15 ms; field of view = 170×170 mm2; 240 slices with slice thickness of 0.53 mm; voxel size = 0.53×0.53×0.53 mm3; and scan time = 8 min, both before and after contrast agent injection. Hyperintense plaques (HIP), plaque surface irregularity, the degree of lumen stenosis, the degree of enhancement, and the average number and length of visualized LSA branches were measured. A statistical analysis of these parameters between the A-to-A embolism and BAD groups was conducted.Results
Fifty-eight first-time stroke patients were enrolled, including 32 with A-to-A embolism and 26 with BAD. HIPs and plaque surface irregularity were more frequently observed in the A-to-A embolism group than in the BAD group (P < 0.001 and P = 0.028, respectively). Patients with A-to-A embolism infarction had a more severe degree of stenosis than patients with BAD infarction (P < 0.001). The average number and lengths of the visualized LSA branches were more significantly reduced in the patients with BAD compared with A-to-A embolism (P = 0.017 and P = 0.029, respectively). Logistic regression analysis showed that HIP and more LSA branches were strongly associated with A-to-A embolism, with odds ratios of 12.21 and 2.07 (95% confidence interval 2.41 – 61.83 and 1.06 – 4.07; P = 0.002 and 0.034), respectively.Discussion
Previous studies have indicated that there is an association between HIP and lesion instability in coronary plaques. 8 However, a few studies of intracranial vessel wall imaging have reported that vulnerable plaque is associated with HIP. 9 Our current study showed that HIP was more frequent in patients with A-to-A embolism than BAD and that it was also an independent predictive factor for A-to-A embolism infarction. Furthermore, we observed that HIP on the surface of the plaques, which appears to be in situ thrombosis, could be the reason for the increased risk of A-to-A embolism. We also found that patients with A-to-A embolism had a higher frequency of plaque surface irregularity and HIP together, suggesting that the plaque surface irregularity of the intracranial arteries may increase the risk of formation of surface thrombus. Furthermore, patients with BAD in our study were shown to have a significant reduction in both the number and lengths of LSA branches on the affected sides. This may be due to the occlusion occurring at the orifice of the LSA branches caused by parent artery plaque, resulting in a decrease in blood flow and thrombosis in these small arteries.Conclusion
A-to-A embolism infarction has a distinct vascular pathophysiology in terms of plaque and LSA characteristics compared with BAD. WB-HRMRI enables the combination of vessel wall and LSA imaging, which together can predict future stroke.Acknowledgements
No acknowledgement found.References
1. Wong KS, Gao S, Chan YL, et al. Mechanisms of acute cerebral infarctions in patients with middle cerebral artery stenosis: a diffusion-weighted imaging and microemboli monitoring study. Ann Neurol. 2002;52:74-81.
2. Bang OY, Heo JH, Kim JY, et al. Middle cerebral artery stenosis is a major clinical determinant in striatocapsular small, deep infarction. Arch Neurol. 2002;59:259-263.
3. Ryoo S, Lee MJ, Cha J, et al. Differential Vascular Pathophysiologic Types of Intracranial Atherosclerotic Stroke: A High-Resolution Wall Magnetic Resonance Imaging Study. Stroke. 2015;46:2815-2821.
4. Kim JM, Jung KH, Sohn CH, et al. Middle cerebral artery plaque and prediction of the infarction pattern. Archives of neurology 2012;69:1470-1475.
5. Zhao DL, Deng G, Xie B, et al. Wall characteristics and mechanisms of ischaemic stroke in patients with atherosclerotic middle cerebral artery stenosis: a high-resolution MRI study. Neurological research 2016;38:606-613.
6. Fan Z, Yang Q, Deng Z, et al. Whole-brain intracranial vessel wall imaging at 3 Tesla using cerebrospinal fluid-attenuated T1-weighted 3D turbo spin echo. Magnetic resonance in medicine 2017;77:1142-1150.
7. Yang Q, Deng Z, Bi X, et al. Whole-brain vessel wall MRI: A parameter tune-up solution to improve the scan efficiency of three-dimensional variable flip-angle turbo spin-echo. Journal of magnetic resonance imaging : JMRI 2017; DOI: 10.1002/jmri.25611.
8. Kolodgie FD, Gold HK, Burke AP, et al. Intraplaque hemorrhage and progression of coronary atheroma. The New England journal of medicine. 2003;349:2316-2325
9. Xu WH, Li ML, Gao S, et al. Middle cerebral artery intraplaque hemorrhage: Prevalence and clinical relevance. Annals of neurology. 2012;71:195-198
Figures