0861
High-Resolution, Non-contrast Pseudo-Continuous Arterial Spin-Labeling (PCASL) MR-Angiography compared to standard clinical MRI in the detection of intracranial vessel pathologies with emphasis on intracranial arteriovenous shunts.1Radiology, Basel University Hospital, Basel, Switzerland, 2University of Wisconsin Madison, Madison, WI, United States, 3William Beaumont Hospital, Royal Oak, MI, United States
Synopsis
The detection of intracranial AV-shunts may be difficult with non-invasive imaging. Due to the nature of spin-labeled protons, ASL-based MRA is likely to be highly specific for AV-shunting. We sought to determine the sensitivity and specificity of ASL-based MRA in a group of 32 patients, among those 14 with AV-shunts. Furthermore, the diagnostic performance for vascular pathology not associated with AV-shunting was assessed. We found ASL-based MRA to be more specific with an equivalent sensitivity compared to a clinical MRA-exam for intracranial AV-shunts. All vascular pathology not associated with AV-shunting were detected with ASL-based and clinical MRA.
Introduction
Arteriovenous malformations (AVMs) and arteriovenous fistulas (AVFs) can be challenging to diagnose with noninvasive imaging. ASL-perfusion images were shown to increase the sensitivity and specificity in the detection of AV-shunting when applied as an adjunct to a clinical MR examination 1. However, ASL perfusion sequences do not provide angiographic information due to the low spatial resolution. ASL-based MR-angiography might be suited to address both needs: (1) detection and (2) angiographic information of vascular pathologies and should theoretically have a high sensitivity for AV-shunts 2. In this study, we assessed the sensitivity and specificity of high-resolution, Pseudo-Continuous Arterial Spin-Labeling (PCASL) magnetic resonance angiography (MRA) with 3D-radial acquisition for the detection of intracranial arteriovenous (AV)-shunts and furthermore evaluated the diagnostic performance for additional vascular pathologies.Materials and Methods
32 patients that underwent PCASL-MRA, clinical MRI-exam and DSA were included in this analysis. 12 patients presented with arteriovenous malformations (AVM, n=8) and arteriovenous fistulas (AVF, n=4). Of the remaining 20 patients, 10 presented with untreated aneurysms, 7 with treated aneurysms and 1 with high-grade MCA stenosis. The patients without AV-shunting were included as a negative control group. The clinical MRI included 3D time-of-flight MRA in all, and time-resolved, contrast-enhanced MRA in 9 cases (6 cases with AV-shunting). PCASL-MRA and clinical MR-imaging were independently evaluated by two neuroradiologists blinded to patient history. For the detection of pathologies not associated with AV-shunting (aneurysms, stenosis), sensitivity and specificity was calculated for both PCASL-MRA and clinical MRI.
To avoid off-resonance artifacts that are a particular concern in intracranial applications of PCASL due to susceptibility at the skull base and nasal fossa, the acquisition module consists of a low flip angle SPGR readout combined with an undersampled 3D radial sampling strategy. The PCASL acquisition has been described in detail 3. PCASL-vastly undersampled isotropic-voxel radial projection imaging (VIPR) parameters include: labeling duration 3 s; image acquisition window 1 s; FOV 22x22x16 cm3; acquired 3D isotropic resolution 0.68 mm; readout bandwidth +/-62.50 kHz; TR/TE=5.06/1.07ms; fractional echo 0.75; flip angle 10°; no flow compensation. A total of 12,000 projections were collected in a scan time of 8:27 minutes.
Results
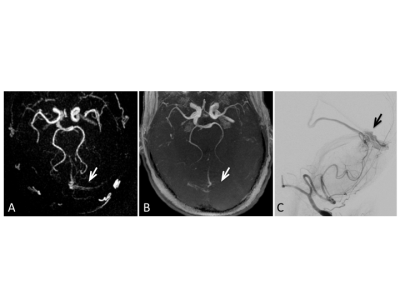
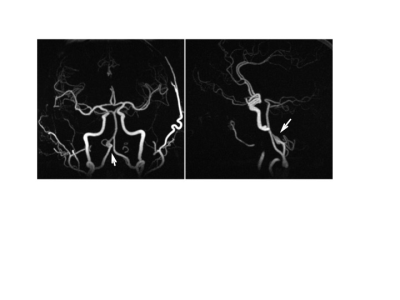
With PCASL-MRA, 12 out of 12 cases with AV-shunting were correctly identified by reader 1 and 11 out of 12 cases with AV-shunting were correctly identified by reader 2. 1 AVM-case was rated false-negative by reader 2. 18 out of 18 cases without AV-shunting were correctly identified as negative for AV shunting by both readers. This leads to a sensitivity of 100% and a specificity of 100% for reader 1 and a sensitivity of 91.7% and a specificity of 100% for reader 2 for the diagnosis of AV-shunts. Based on clinical MRA, 11 out of 12 cases with AV-shunting were correctly identified by both readers. 1 AVM-case was rated false negative by both readers. 1 case was rated false positive by reader 1. 17 out of 18 cases were correctly identified as negative for AV shunting by reader 1 and 18 out of 18 by reader 2. This leads to a sensitivity of 91.7% and a specificity of 94.4% for reader 1 and a sensitivity of 91.7% and a specificity of 100% for reader 2. 10 out of 10 untreated aneurysms were correctly identified with PCASL-MRA by both readers (sensitivity: 100%, specificity: 100%) as well as with clinical MRA (sensitivity: 100%, specificity: 100%). One case with a high grade intracranial stenosis was correctly identified by both reader with PCASL-MRA and clinical MRA.Discussion
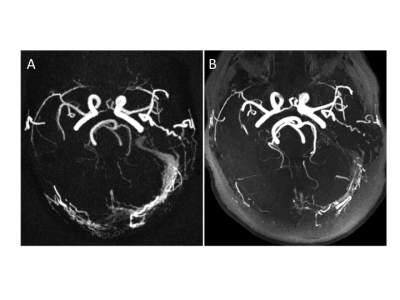
PCASL-MRA proved to be equivalent or superior compared to a clinical MRA-exam including a ToF-MRA in all cases and time resolved, contrast-enhanced MRA in 50% of patients with AV-shunts in the detection of intracranial AV-shunting. This finding supports the hypothesis that if a direct communication between the arterial and the venous system exists, the labeled arterial spins are able to carry over signal into the venous vasculature. Therefore, venous signal in a PCASL angiogram is likely to reflect arterio-venous shunting with high specificity 4. Without the presence of AV-shunting, the excited protons undergo an exchange with water protons contained in the extracellular space, leading to a rapid signal loss within the capillary bed and consecutively a highly specific display of the arterial system without venous contamination (Fig 3). In addition, all additional vascular pathologies were detected with PCASL-MRA equivalent to clinical MRA.Conclusion
Non-contrast PCASL-MRA with 3D-radial acquisition is a powerful tool for the detection and characterization of intracranial arteriovenous shunts with a sensitivity and specificity equivalent or higher than routine clinical MRI.Acknowledgements
Jenelle Fuller, Sara John, Kelli Hellenbrand for their help with MR-scanningReferences
1) Wolf RL, Wang J, Detre JA, et al. Arteriovenous shunt visualization in arteriovenous malformations with arterial spin-labeling MR imaging. AJNR Am J Neuroradiol. 2008;29(4):681-7.
2) Amukotuwa SA, Heit JJ, Marks MP, et al. Detection of Cortical Venous Drainage and Determination of the Borden Type of Dural Arteriovenous Fistula by Means of 3D Pseudocontinuous Arterial Spin-Labeling MRI. AJR Am J Roentgenol. 2016;207(1):163-9.
3) Wu H, Block WF, Turski PA, et al. Noncontrast-enhanced three-dimensional (3D) intracranial MR angiography using pseudocontinuous arterial spin labeling and accelerated 3D radial acquisition. Magn Reson Med. 2013;69(3):708-15.
4) Raoult H, Bannier E, Robert B, et al. Time-resolved spin-labeled MR angiography for the depiction of cerebral arteriovenous malformations: a comparison of techniques. Radiology. 2014;271(2):524-33.
Figures