0852
GABA levels in children with Autism and typically developing children differentially relate to social gesture performance1Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 3Department of Radiology, University of Calgary, Calgary, AB, Canada, 4Department of Neurology, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 5Center for Neurodevelopmental and Imaging Research, Kennedy Krieger Institute, Baltimore, MD, United States
Synopsis
Children with Autism (ASD) often suffer from motor abnormalities and the main inhibitory neurotransmitter GABA has been linked to ASD. We measure GABA in the primary sensorimotor cortex using MRS and measure praxis (social gesturing) in children with ASD and healthy children (TDC). We show that GABA is differentially related to social gestures in children with and without ASD. This suggests a complex relation between inhibition and gesture function. Reduced GABA levels may impair the performance of gestures with a communicative purpose, contributing to autistic phenotypes. Understanding the GABA system in ASD is important for developing patient-specific treatment in ASD.
Purpose
Autism Spectrum Disorder (ASD) is characterized by impairments in social cognition, communication, and repetitive behaviors1. Motor abnormalities are common in ASD and have been linked to social and communicative features2. Praxis - the performance of complex gestures with a communicative or functional purpose, has been shown to be impaired in ASD3. The inhibitory neurotransmitter GABA plays an important role in regulating motor behaviors and is implicated in ASD pathophysiology4, but the link between GABA and autism-related dyspraxia is unclear. Here, we measure GABA levels in the sensorimotor cortex of typically developing children and children with ASD using edited MRS and we evaluate praxis. We hypothesize that children with ASD show worse praxis, and that better praxis performance is correlated with higher sensorimotor GABA levels.Methods
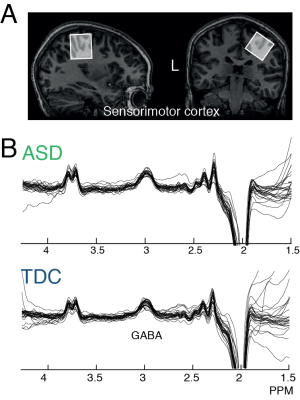
Subject and parental consent were obtained under local IRB approval. Eligibility: Children with ASD met the DSM-V criteria for ASD, confirmed using the Autism Diagnostic Observation Schedule-Version 2 (ADOS-2). All Typically Developing Children (TDC) were free of-psychiatric disorders. Data were acquired in 24 children with ASD (10.52 ± 1.25 years, 6F) and 26 TDC (10.11 ± 1.38 years; 8F) with normal IQ. Imaging: GABA-edited MR spectra were acquired from (3 cm)3 voxels over the right primary sensorimotor cortex (M1; Fig. 1A&B) using the MEGA-PRESS sequence5 on a Philips 3T ‘Achieva’ scanner (Philips Medical Solutions, Best, the Netherlands) with a 32-channel head coil: TE/TR 68/2000 ms; 14 ms editing pulse at 1.9 (ON) and 7.46 (OFF) ppm, 320 transients (10 min scan). A T1-weighted structural image was acquired for voxel placement. GABA levels are quantified relative to the unsuppressed water signal from the same voxel and tissue corrected6, as implemented within Gannet7 v2.1. Praxis: A pediatric version of the Florida Apraxia Battery (FAB) was used to examine performance of skilled gestures, known to robustly discriminate children with ASD and TDC. Total percent correct and total error scores were used. Statistics: Student’s T-tests were used to assess differences in praxis performance and GABA levels. Pearson correlations were used to assess the correlation between performance and GABA levels. Fisher’s r-to-z was used to assess the difference in correlation coefficients between cohorts.Results
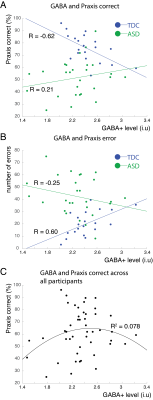
Mean praxis percentage correct (ASD; 52.1 ± 15.21, TDC; 75.93 10.94, p < 0.001) and total errors (ASD; 42.3 ± 15.95, TDC 20.57 ± 9.87, p < 0.001) differed significantly between cohorts. GABA values were significantly lower in ASD (ASD; 2.24 ± 0.38, TDC; 2.4 ± 0.25; p < 0.05), as previously shown8. TDC showed a significant negative correlation with praxis percentage correct scores (-0.62, p = < 0.01), whereas there was trend towards a positive correlation in children with ASD (0.21, p = 0.12); Figure 2A). TDC showed a positive correlation between GABA and praxis errors (R – 0.6, p < 0.001) and children with ASD showed a trend towards a negative correlation (R = -0.25, p = 0.1; Figure 2B). The correlations were significantly different between cohorts for both praxis percentage correct and error (Fisher r-to-z, for both p < 0.01). We explored whether an inverted u-shape (quadratic fit) fit the data across all participants (Figure 2C), but significance did not reach alpha of 0.05 (R2 = 0.078, p =0.06). There were no significant differences in MRS data quality.Discussion
Our data show that GABA is differentially related to praxis performance in children with ASD and TDC. This finding is surprising, given that in previous work we showed that more GABA in regions related to a task is generally better8. It is not quite clear how to interpret these results, but the correlations are robust in TDC. Additional resting-state fMRI, or left hemisphere GABA levels, might elucidate this relationship. Within TDC, increased GABA levels in M1 may reduce gesture performance, by suppressing SMA/PMC function to properly plan and execute movements, whereas in ASD, pathologically-reduced GABA may hinder efficient encoding of motor commands. There is a subset of children with ASD9 who show reduced GABA levels and are most severely affected in gesture performance. While not significant, the quadratic fit suggests there may be a u-shaped relationship where there is an ‘optimum’ in the GABA levels to regulate motor control.Conclusion
Children with ASD show reduced GABA levels, whichare associated with abnormalities in performance on gesture tasks, which is associated with socially motivated motor control. Reduced GABA levels may ultimately impair the performance of complex gestures with a communicative or functional purpose, contributing to subgroups within the autistic phenotype. Understanding the GABA system in ASD is important for developing future and novel targets for patient-specific treatment in ASD.Acknowledgements
This work was supported by NIH P41 EB015909, R21 MH098228, R01 MH078160, and R01 EB016089. NAJP is supported by K99 MH107719References
1. Rogers, S. J. & Ozonoff, S. Annotation: what do we know about sensory dysfunction in autism? A critical review of the empirical evidence. J. Child Psychol. Psychiatry Allied Discip. 46, 1255–1268 (2005).
2. Mostofsky, S. H. et al. Developmental dyspraxia is not limited to imitation in children with autism spectrum disorders. J. Int. Neuropsychol. Soc. 12(3), 314-26 (2006)
3. Mostofsky, S. H. & Ewen, J. B. Altered Connectivity and Action Model Formation in Autism Is Autism. Neurosci. 17, 437–448 (2011).
4. Rubenstein, J. L. & Merzenich, M. M. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav 2, 255–267 (2003).
5. Mescher, M., Merkle, H., Kirsch, J., Garwood, M. & Gruetter, R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 11, 266–272 (1998).
6. Harris, A. D., Puts, N. A. J. & Edden, R. A. E. Tissue correction for GABA-edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. J. Magn. Reson. Imaging 42, 1431–1440 (2015).
7. Edden, R. A., Puts, N. A., Harris, A. D., Barker, P. B. & Evans, C. J. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J. Magn. Reson. Imaging 40, 1445–1452 (2014).
8 Puts, N. A. J., Edden, R. A. E., Evans, C. J., McGlone, F. & McGonigle, D. J. Regionally Specific Human GABA Concentration Correlates with Tactile Discrimination Thresholds. J. Neurosci. 31, 16556–16560 (2011).
9 Puts, N. A. J. et al. Reduced GABA and altered somatosensory function in children with autism spectrum disorder. Autism Res 10, 608–619 (2016).
Figures