0850
Reduced Nasal Nitric Oxide Predicts Mis-matched Alterations in Regional Cerebral Perfusion and Functional Brain Connectivity in Older Children with Congenital Heart Disease1Radiology, Children's Hospital of Pittsburgh, Pittsburgh, PA, United States, 2Children's Hospital of Pittsburgh, Pittsburgh, PA, United States
Synopsis
We investigate the relationship between congenital heart disease (CHD), regional CBF, functional connectivity, and nasal nitric oxide (nNO) levels in older children. Low nNO is associated with poor cardiac function in CHD although the precise mechanism is unknown. Results show that, in CHD patients, reduced nNO is associated with reduced regional CBF in the salience and default mode networks as well as reduced segregation globally (modularity) and regionally (frontally, parietally, and subcortically); these relationships are not present in normal controls. These results suggest intrinsic brain deficits (more so than impaired substrate delivery) may underlie neurocognitive deficits in CHD patients.
Introduction
Poor neurocognitive outcome in congenital heart disease (CHD) is thought to be caused by abnormal cerebral substrate delivery secondary to poor cardiac function, associated with low levels of nasal nitric oxide (nNO)1, 2. nNO may be easily and non-invasively measured, and thus may serve as a proxy for systemic NO. As such, we tested the hypothesis that reduced nNO would predicted matched patterns of reduced global cerebral perfusion (ASL) and functional brain connectivity (fcMRI-BOLD) in CHD older children as compared to controls.3, 4Methods
ASL: Pseudo-continuous ASL (pCASL) data was successfully acquired from 19 CHD subjects (15 M, 4 F, age = 13.47 +/- 2.67 years) and 25 control subjects (13 M, 12 F, age = 13.38 +/- 2.85 years) on a Siemens Skyra 3T system. Control-tag and average control images were computed using a voxelwise GLM, with motion and drift parameters included as nuisance covariates; absolute CBF was estimated from the fractional difference using the two-compartment model5 with literature values6. The reference image was segmented into GM in native space and spatially normalized into MNI space using the gray matter template in SPM8; the CBF map was spatially normalized using the same transformation.
fcMRI: Resting-state fMRI (rs-fcMRI) was successfully obtained from 13 CHD subjects (10 M, 3 F, age = 13.42 +/- 2.92 years) and 11 controls (4 M, 7 F, age = 13.3 +/- 2.70 years). The method of Power et al.7, 8 was used to minimize the risk of spurious results due to participant motion; Using the AAL template, 90-X-90 correlation matrices were computed and thresholded either at fixed values of cost or at fixed values of correlation threshold. Graph analysis metrics were computed using Brain Connectivity ToolBox and in-house routines in IDL.
nNO: Nasal nitric oxide (nNO) values were acquired using tidal breath sampling with a CLD 88sp NO analyzer obtained via low continuous suction at a rate of 0.3 L/min from each naris using a nasal olive sampling cannula during tidal breathing. Each naris was sampled once for 50 s. Five peak inflections from nNO concentration curve were analyzed against the sampling flow and averaged to yield a final value in nl/min.
fcMRI/nNO Association: A mixed-model GLM using an unstructured correlation matrix was performed with nNO and the nNO-X-CHD interaction as variables of interest and participant age at scan, age at nNO collection, and sex included as covariates of no interest.
CBF/nNO Association: A voxelwise GLM was performed as above including only participants with rms translational motion < 1 voxel and voxels with GM probability > 78%. Additionally, rms motion and GM probability (different for each voxel) were included as covariates of no interest. Multiple comparisons were corrected for using a Monte Carlo approach9.
Results
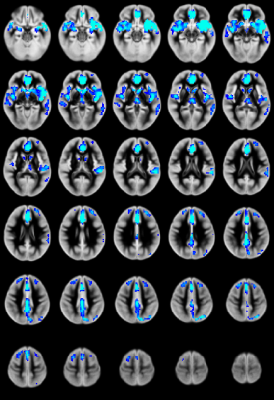
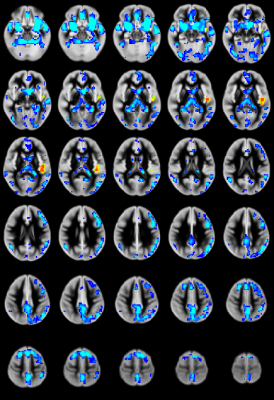
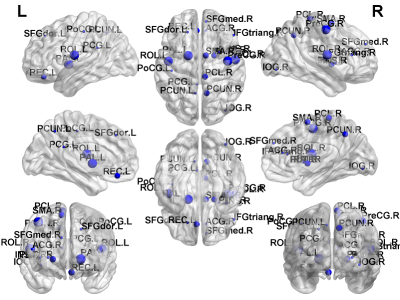
CBF: Significant CHD-X-nNO interactions were seen for CBF regionally in the salience and default mode networks (Figure 1). For the CHD cohort, reduced nNO predicted increased regional CBF mainly in the salience and default mode networks (Figure 2); no significant results were found for the control cohort.
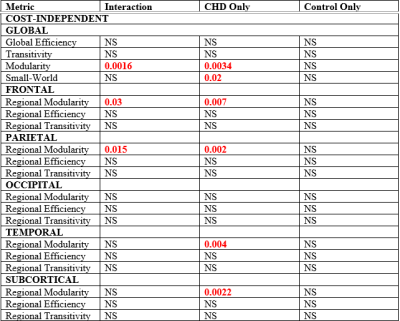
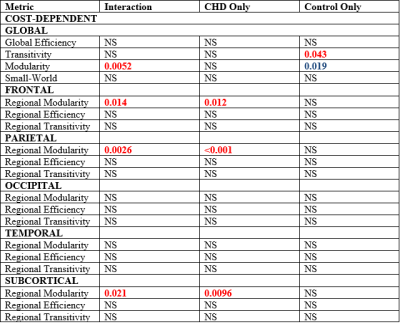
fcMRI: Highly significant CHD-X-nNO interactions were seen for Louvain modularity, as well as regional modularity frontally, parietally, and subcortically (Figure 3, 4). In the CHD cohort, low nNO predicted reduced brain network segregation (Louvain modularity as well as regional modularity frontally, parietally, temporally, and subcortically); in the control cohort, a significant negative association was seen for Louvain modularity. For the CHD cohort, reduced nNO predicted increased participation coefficient (reduced modularity) in frontal, parietal, and subcortical nodal regions (Figure 5).
Discussion
Given that reduced nNO is a predictor of poor cardiac function and clinical outcomes in CHD, we had anticipated that nNO would have predicted reduced global CBF and functional brain connectivity in matched brain regions. However, our results showed that reduced nNO is associated with hyperperfusion localized to very specific regions (salience and default mode). In contrast, lower nNO predicts abnormal global functional connectivity (reduced functional segregation) regionally mismatched to the nNO - ASL perfusion results. NO is known to be a key mediator of neuro-vascular coupling (NVC), produced from neuronal nitric oxide synthase (nNOS)10 or endothelial nitric oxide synthase (eNOS)11, even though nNO may be more reflective of inducible NOS (iNOS).Conclusion
Altered NO metabolism is associated with mismatched regional alterations in cerebral perfusion and functional connectivity in CHD patients. These findings suggest that intrinsic brain deficits (neuronal vascular coupling/brain metabolism) may be important mediators of neurocognitive deficits in CHD patients (relative to impaired cerebral substrate delivery from cardiac dysfunction).Acknowledgements
No acknowledgement found.References
1. Stewart E et al., J Thorac Cardiovasc Surg, 2017.
2. Adams P et al., J Am Heart Assoc, Accepted, 2017.
3. Schmithorst VJ, Panigrahy A, ISMRM Annual Meeting, Honolulu, HI, 901, 2017.
4. Schmithorst VJ, Panigrahy A, ISMRM Annual Meeting, Honolulu, HI, 1079, 2017.
5. Alsop DC, Detre JA, J Cereb Blood Flow Metab, 16, 1236-49, 1996.
6. Schmithorst VJ et al., J Magn Reson Imaging, 39, 1104-17, 2014.
7. Power JD et al., Neuroimage, 84, 320-41, 2014.
8. Power JD et al., Neuroimage, 105, 536-51, 2015.
9. Ledberg A et al., Neuroimage, 8, 113-28, 1998.
10. Stefanovic B et al., Journal of Cerebral Blood Flow & Metabolism, 27, 741-54, 2006.
11. Stobart JLL et al., Proceedings of the National Academy of Sciences, 110, 3149-54, 2013.
Figures