0849
Macroscopic Hyperdynamic CSF and Ciliary Motion Dysfunction Predict Executive Dysfunction in Children and Adolescents with Congenital Heart Disease1Radiology, University of Pittsburgh, Pittsburgh, PA, United States, 2Radiology, Children's Hospital of Pittsburgh of UPMC, Pittsburgh, PA, United States, 3Pediatrics Internal Medicine, Children's Hospital of Pittsburgh UPMC, Pittsburgh, PA, United States, 4Developmental Biology, University of Pittsburgh, Pittsburgh, PA, United States, 5Department of Physical Medicine & Rehabilitation, Children's Hospital of Pittsburgh UPMC, Pittsburgh, PA, United States, 6Biomedical Informatics, University of Pittsburgh, Pittsburgh, PA, United States
Synopsis
An important role for cilia in congenital heart disease (CHD) pathogenesis has been seen in mouse model of CHD. There is a high prevalence of motile respiratory cilia dysfunction in human CHD patients. In this study, we investigate whether abnormal respiratory cilia motion in preadolescent CHD patients may be correlated with alterations in macroscopic CSF flow dynamics and poor executive cognitive function using phase contrast imaging. We show disturbance of CSF flow dynamics is significantly correlated with neurocognitive impairment in CHD subjects.
INTRODUCTION:
An important role for cilia in congenital heart disease (CHD) pathogenesis has emerged from a recent large-scale mouse mutagenesis screen for mutations causing CHD.1 We previously showed a high prevalence of motile respiratory cilia dysfunction in CHD patients. As motile cilia are also known to mediate CSF flow in the brain ependyma via a temporospatially regulated network,2 we hypothesize CHD patients with respiratory ciliary dysfunction may also have ependymal ciliary motion defects that can impact macroscopic CSF flow dynamics and cause increased volume of extra-axial fluid. Indeed, we recently showed increase volume of frontal-temporal cerebral spinal fluid (CSF) in CHDs infants is associated with aberrant respiratory ciliary motion. Hence in this study, we will investigate whether abnormal respiratory cilia motion in preadolescent CHD patients may be correlated with alterations in macroscopic CSF flow dynamics and poor executive cognitive function.METHODS:
A total of 102 children/adolescent participants (CHD=45, 14.4±5.95 y.o.; Healthy Controls=57, 14.4±4.03 y.o.) were prospectively recruited, with 57 (CHD=23; Healthy Controls=34) having analyzable CSF flow MRI scans, and 17 (CHD=10; Controls=7) had nasal scrape for ciliary motion (CM) assessment.3 The CM was scored by three experts using a four-point scale. A Siemens 3T Skyra system/32-channel head coil with through-plain CSF flow acquired using phase contrast gradient echo at the level of the cerebral aqueduct with the following parameters: velocity encoding=12 cm/s; TR/TE=9.66/30.40 ms; flip angle=15°; matrix=256x256; in plane resolution=0.6mmx0.6mm; slice thickness=5mm;). A circular ROI encompassing the lumen and wall of the aqueduct was used for each study with the surrounding brain parenchyma used as reference. The following CSF flow metrics were calculated using ARGUS software: Average Velocity (AV), Average Flow over Range of Scan (TAF), Average Flow per Minute (AFpM), Total Forward Volume (TFV), Total Reverse Volume (TRV), Net Forward Volume (NFV). Cognitive assessments of processing speed and executive functioning included subtests from the Delis-Kaplan Executive Functioning System (D-KEFS), Wechsler Intelligence Scale for Children, 4th Edition (WISC-IV), and NIH Toolbox (NIHTB). Analyses using general linear model were conducted using SAS.4RESULTS:
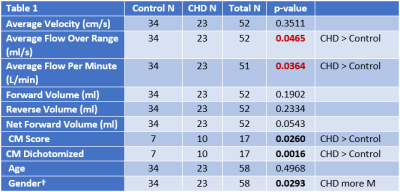
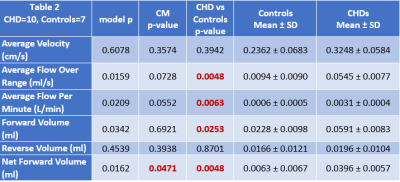
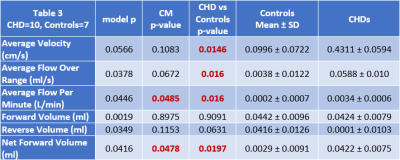
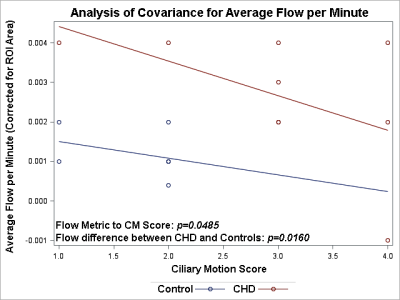
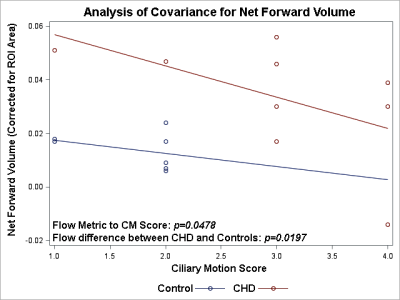
The children/adolescent CHD group compared to healthy controls demonstrated no difference in age, but had higher prevalence of males (p=0.0293) and of ciliary motion abnormality (p=0.0260). Compared to healthy controls, the CHD group demonstrated hyperdynamic CSF flow with greater average CSF flow over the range of the scan (p=0.0465) and increased average flow per minute (p=0.0364) (Figure 1). Using multiple regression analysis with the incorporation of CM score, additional differences between CHD and controls were noted including TAF (p=0.0048), AFpM (p=0.0063), TFV (p=0.0253), and NFV (p=0.0048), consistent with hyperdynamic flow in the CHD group, with increased CM abnormality predicting the opposite effect (reduced CSF flow) (Figure 2). In contrast, only NFV was found to have a significant association with CM (p=0.0471) (Figure 2). After correcting for ROI area, the CHD group still demonstrated hyperdynamic CSF flow characteristics compared to controls (Figure 3). However, increased CM abnormality predicted reduced CSF flow metrics including decreased AFpM (Figure 4) and decreased NFV (Figure 5). CHD subjects exhibited impaired processing speed (p=0.0006;) and impaired executive function such as inhibition of prepotent responses (p=0.0036), cognitive flexibility (p=0.0109), sequencing (p<0.0001). Increased CSF Average Flow per Minute predicts reduced processing speed (p=0.0137), as well as impaired executive function – working memory (p=0.0481). Similarly, abnormal ciliary motion scores predict reduced processing speed (g p=0.0152;) and impaired working memory component of executive function (p=0.0399).DISCUSSION:
We observed macroscopic CSF flow is increased in CHD patients compared to controls and this is correlated with neurocognitive impairment. This is reminiscent of hyperdynamic flow seen in normal pressure hydrocephalus (NPH) with increased brain tissue compliance and impedance to CSF flow associated with white matter abnormality, and also communicating hydrocephalus linked to primary cilia defects.5 Interestingly, abnormal ciliary motion in CHD patients was found to be predictive of reduced CSF flow metrics and this was also correlated with neurocognitive impairment.6 These findings provide the first evidence suggesting microscopic motile cilia driven flow at the brain ependymal surface may impact bulk CSF flow, a possibility that warrant further investigation.CONCLUSION:
Together these findings show disturbance of CSF flow dynamics is significantly correlated with neurocognitive impairment in CHD subjects. This is impacted by motile cilia dysfunction that may involve the interaction of microscopic flow at the ependymal surface with bulk CSF flow.Acknowledgements
No acknowledgement found.References
1. Li, Y., Klena, N.T., Gabriel, G.C., Liu, X., Kim, A.J., Lemke, K., Chen, Y., Chatterjee, B., Devine, W., Damerla, R.R. and Chang, C., 2015. Global genetic analysis in mice unveils central role for cilia in congenital heart disease. Nature, 521(7553), pp.520-524.
2. Faubel, Regina, Christian Westendorf, Eberhard Bodenschatz, and Gregor Eichele. "Cilia-based flow network in the brain ventricles." Science 353, no. 6295 (2016): 176-178.
3. Panigrahy, Ashok, Vincent Lee, Rafael Ceschin, Giulio Zuccoli, Nancy Beluk, Omar Khalifa, Jodie K. Votava-Smith et al. "Brain dysplasia associated with ciliary dysfunction in infants with congenital heart disease." The Journal of pediatrics 178 (2016): 141-148.
4. SAS Institute Inc., SAS 9.4, Cary, NC: SAS Institute Inc., 2014.
5. Carter, Calvin S., Timothy W. Vogel, Qihong Zhang, Seongjin Seo, Ruth E. Swiderski, Thomas O. Moninger, Martin D. Cassell et al. "Abnormal development of NG2+ PDGFR-[alpha]+ neural progenitor cells leads to neonatal hydrocephalus in a ciliopathy mouse model." Nature medicine 18, no. 12 (2012): 1797-1804.
6. Chang, L. H., C. Y. Chen, M. Y. Chen, T. Y. Huang, and H. W. Chung. "Normal and abnormal cerebrospinal fluid dynamics evaluated by optimized cine phase-contrast MR imaging." Chin J Radiol 25 (2000): 191-195.
Figures