0832
MRI of whole gut transit time in newly diagnosed coeliac disease.1Nottingham Digestive Diseases Centre and NIHR Nottingham Biomedical Research Centre, Nottingham University Hospitals NHS Trust and University of Nottingham, Nottingham, United Kingdom, 2Medical Physics and Clinical Engineering, Nottingham University Hospitals, Queen’s Medical Centre, Nottingham, United Kingdom, 3Nottingham NHS Treatment Centre, Nottingham University Hospitals, Queen's Medical Centre Campus, Nottingham, United Kingdom, 4Department of Medicine and Surgery, Scuola Medica Salernitana Università di Salerno, Salerno, Italy
Synopsis
Coeliac disease (CD) is an autoimmune disease which affects 1 in 100 people. There is no cure and the only treatment is a lifelong gluten free diet. This study aims to improve our understanding of the functional motility disorder associated with CD using MRI. Whole gut transit time (WGTT), measured using MRI transit markers, was significantly delayed in the coeliac patients compared to helathy controls (p<0.04). The MRI gut transit test is quick and is acceptable to patients and could help long term monitoring and follow up, complementing existing more invasive techniques.
INTRODUCTION
Coeliac disease is an autoimmune disease which primarily affects the small bowel mucosa resulting in villous inflammation. It is induced in genetically susceptible individuals after ingestion of gluten 1. The only treatment is a life-long gluten free diet (GFD), which results in recovery of the small bowel mucosa and reversal of the enteropathy. Studies involving manometric, breath test and camera pill measurements have independently observed an underlying gastrointestinal (GI) motility disorder in coeliac disease 2. Particularly, oro-coecal transit time seems to be delayed 3, 4. In untreated coeliac patients these GI motor disorders tend to resolve on gluten free diet treatment. Recent MRI work has validated against radiopaque markers a method to measure whole gut transit time (WGTT) exploiting MRI to localize inert transit marker capsules 5. These novel measurements may provide a useful new method to follow up the response to the diet treatment. Here we aimed to use this new non-invasive MRI method to measure WGTT in a group of recently diagnosed coeliac patients and compare their WGTT to a group of healthy controls.METHODS
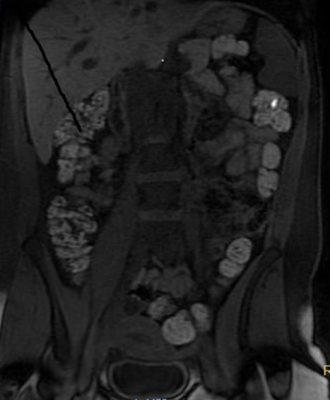
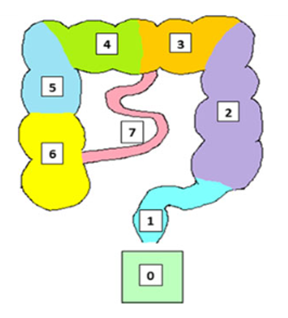
This study was approved by the National Research Ethics Service (REC number 15/LO/0253); all participants gave written informed consent. Patients were eligible for the study if on a gluten-containing diet, and with a duodenal biopsy showing villous atrophy. The non- coeliac healthy volunteers were matched for age and sex. 35 newly diagnosed coeliac patients, 25 females, 10 males aged 20-71, and 25 healthy volunteers, 10 male, 15 female aged 20-65, were enrolled in the study. All MRI scans were acquired in a GE 1.5 Tesla HDX MRI scanner (General Electric Medical Systems, Milwaukee, WI) using a dedicated 12 channel phased array body imaging coil. Participants swallowed five MRI marker capsules 24 hours before undergoing the MRI scan. The capsules (Figure 1) are filled with 0.4 mL water doped with 15 μM Gadoteric acid (Gd-DOTA). These capsules appear bright on T1 imaging (Figure 2) which allows to determine their position within the bowel easily. Participants were asked to fast overnight. On the study day, following a localizing 3 plane scan, a 3D breathhold T1-weighted fat-suppressed (TR 4 ms, TE 2 ms, Field of View 480 mm and 320 x 192 matrix with a 2.6mm slice thickness Liver Acquisition with Volume Acceleration- LAVA) coronal sequence was used to locate the transit capsules. From the images a transit score was then calculated according to each capsule’s position 24 hours after ingestion 5. This scoring system subdivides the colon into eight sections (see figure 3) . Using a previously validated method 5 in which the formula used to calculate the transit time takes into consideration the spread of the marker capsules position along the gut by looking at the difference of each capsule position from the median capsule position, using this to apply a weighting factor to each capsule score. This yields a Weighted Average Position Score (WAPS) for each participant. The WAPS can subsequently be converted to an approximate transit time in hours by extrapolating the validation fit from the Chaddock et al paper 5. Statistical analysis was carried out using Prism 5 (GraphPad Software Inc, San Diego, CA, USA). The data are presented as mean±SEM. The distribution of the data was tested using the Shapiro Wilks normality test.RESULTS
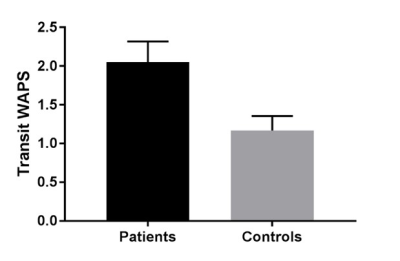
It was possible to calculate a WAPS score from the images for all the participants. The data are shown in Figure 4. In the coeliac patients the mean WGTT was 2.1±0.3, significantly delayed compared to 1.2±0.2 in the healthy controls, p<0.04. Extrapolation the WAPS to transit time in hours results in a mean transit time of 103 hours for the coeliac patients compared to 39 hours for the control HVs.DISCUSSION
The MRI capsule technique to measure WGTT is simple, involving just one visit to the MRI scanner, uses standard sequences and the whole examination takes less than 15 minutes. The images are easily interpreted and examination was well tolerated by participants in the study and does not expose them to ionising radiation. Our data confirms delayed transit in the newly diagnosed coeliac patients. The effect on this of a sustained gluten-free diet remains to be seen.CONCLUSION
This preliminary experience suggests that MRI can offer new insights into the pathophysiology of CD. The imaging exam is quick, non-invasive, uses non-ionising radiation and is acceptable to patients. With the prevalence of CD increasing worldwide and the development of novel non-dietary treatments such as enzyme therapy and therapeutic vaccines 6 new non-invasive measurements of disease severity and progression would therefore be welcome.Acknowledgements
We are grateful for support from the Nottingham Digestive Diseases Centre and NIHR Nottingham Biomedical Research Centre.References
1. Rubio Tapia A. ACG Clinical Guidelines: Diagnosis and Management of Celiac Disease. The American journal of gastroenterology. 2013;108(5):656-76.
2. Pinto-Sanchez MI, Bercik P, Verdu EF. Motility alterations in celiac disease and non-celiac gluten sensitivity. Digestive diseases (Basel, Switzerland). 2015;33(2):200-7. PubMed PMID: 25925923. Epub 2015/05/01. eng.
3. Tursi A. Gastrointestinal Motility Disturbances in Celiac Disease. Journal of Clinical Gastroenterology. 2004;38(8):642-5. PubMed PMID: 00004836-200409000-00004.
4. Sadik R, Abrahamsson H, Kilander A, Stotzer P-O. Gut Transit in Celiac Disease: Delay of Small Bowel Transit and Acceleration after Dietary Treatment. Am J Gastroenterol. 2004 12//print;99(12):2429-36.
5. Chaddock G, Lam C, Hoad CL, Costigan C, Cox EF, Placidi E, et al. Novel MRI tests of orocecal transit time and whole gut transit time: studies in normal subjects. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2014 Feb;26(2):205-14. PubMed PMID: 24165044. Epub 2013/10/30. eng.
6. Crespo Pérez L, Castillejo de Villasante G, Cano Ruiz A, León F. Non-dietary therapeutic clinical trials in coeliac disease. European Journal of Internal Medicine. 2012 1//;23(1):9-14.
Figures