0830
Multiparametric evaluation of inflammation and fibrosis in a radiation-induced murine model of colitis1Laboratory of Imaging Biomarkers, CRI-UMR1149, Inserm, Paris, France, 2Laboratory of Intestinal Inflammation, CRI-UMR1149, Inserm, Paris, France, 3Department of Radiology, Beaujon University Hospital, Clichy, France, 4Department of Pathology, Beaujon University Hospital, Clichy, France, 5Research Laboratory in Radiobiology and Radiopathology, IRSN, Fontenay-aux-Roses, France
Synopsis
The evaluation of fibrosis severity in Crohn’s disease is essential for patient management and prognosis, albeit seldom investigated. We validated a MR approach including diffusion-weighted imaging, magnetization transfer and FAIR perfusion to distinguish between moderate and severe forms of fibrosis in a radiation-induced murine model of colitis. The presence of fibrotic tissue and its accompanying vascular alterations induced a decrease in apparent diffusion coefficient and in perfusion, and an increase in the magnetization transfer ratio. This approach could be applied for diagnosis and assessment of intestinal fibrosis in patients.
Introduction
Crohn’s disease is a chronic idiopathic inflammatory bowel disease presenting continuous coexistence of inflammation and fibrosis at diverse degrees1. In contrast with inflammation, the evaluation of fibrosis severity by imaging techniques and its treatment have been scarcely investigated, whereas fibrosis is the main reason behind Crohn’s disease complications, such as strictures and fistulas, necessitating surgery2. Besides, the severity of fibrosis dictates patient management2.Purpose
Our purpose was to validate a MR approach to evaluate the severity of fibrosis in a radiation-induced murine model of colitis morphologically mimicking Crohn’s disease phenotype. Our approach included conventional T1- and T2-weighted imaging and MR techniques potentially able to assess fibrosis: diffusion-weighted imaging (DWI)3 and magnetization transfer (MT)4. We also investigated the performance of flow-sensitive alternating inversion recovery (FAIR) perfusion for assessing the intestinal microvascular alterations known to occur in Crohn’s disease3.Methods
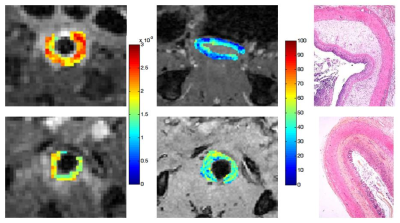
Colitis was induced with localized 27Gy single-dose radiation in 9-week-old rats5. MRI sessions were conducted at 2 weeks (n = 24) and 12 weeks after irradiation (n = 39) and in non-irradiated rats (n = 10) using a 7T Bruker PharmaScan MRI system. One-mm-thick axial slices were acquired with different schemes, starting with fat-suppressed respiratory-gated T2-weighted (TE/TR = 56/5000 ms, 300 µm in-plane resolution) and T1-weighted (TE/TR = 3.8/510 ms, 300 µm in-plane resolution) sequences. A fat-suppressed respiratory-gated DWI spin-echo sequence with echo-planar readout was acquired with TE/TR = 31/2500 ms, 500 µm in-plane resolution, 3 orthogonal directions and 6 b values between 0 and 700 s/mm2. The non-gated FLASH-based MT sequence was initially optimized in vitro on rat colon and muscle tissue with the following parameters: TE/TR = 3.7/374 ms, 250 µm in-plane resolution and 20 ms Gaussian saturation pulse with 5 kHz offset frequency and 20 µT power level. Finally, a non-gated FAIR-RARE perfusion acquisition was implemented with TE/TR = 46/12000 ms, 430 µm in-plane resolution, 1.5 mm imaging slice thickness, 4.5 mm inversion slice thickness and 20 inversion times from 30 to 5730 ms.
For processing, the maximal wall thickness was measured on T2-weighted images, as well as the T1 and T2 signal intensity of the entire wall normalized to muscle signal intensity (T1SInorm and T2SInorm). The apparent diffusion coefficient (ADC) was calculated by fitting the data to a monoexponential model and the MT ratio was calculated using the unsaturated/saturated acquisitions and normalized to the muscle MT ratio (MTRnorm). Finally, perfusion was calculated by determining selective and global R1 from the FAIR-RARE acquisition. Following MRI, animals were euthanized and the distal colon and rectum were extracted for histological analyses. Each sample was scored for inflammation (0-6)6 and fibrosis (0-4)1.
Results
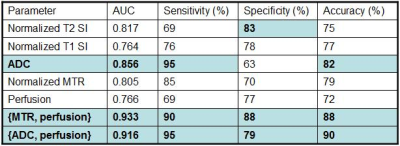
All the MRI parameters were different between non-irradiated and irradiated animals (p < 0.01). Two weeks after irradiation, the colon presented histopathological features of severe inflammation (score 5±1) associated with minimal to moderate submucosal fibrosis (1.5±0.5), while 12 weeks after irradiation, severe transmural fibrosis was observed (3.6±0.5) with similar inflammatory background (5±2). Evolution from submucosal to transmural fibrosis induced a decrease in T2SInorm (4.2±0.7 vs 3.1±1, p < 0.0001), T1SInorm (1.4±0.1 vs 1.3±0.1, p = 0.0007), ADC (2.2±0.4 ×10-3 mm2/s vs 1.7±0.3 ×10-3 mm2/s, p < 0.0001) and perfusion (60±26 ml/min/100g vs 37±21 ml/min/100g, p = 0.001) while MTRnorm increased (0.53±0.11 vs 0.63±0.07, p < 0.0001), which was confirmed in 7 rats with longitudinal follow-up. There was no difference in wall thickness between the 2 groups (Figures 1 and 2). For the classification of fibrosis between submucosal and transmural, the diagnostic performance of the parameters was similar, with areas under the ROC curve (AUROC) between 0.76 and 0.86, and accuracies between 72% and 82% (Table 1). The parameter presenting the highest AUROC and accuracy was ADC (0.86 and 82%, respectively). Logistic regression analysis revealed that the best combinations for classifying fibrosis as submucosal or transmural were {MTRnorm, perfusion} or {ADC, perfusion}, with respective AUROC/accuracy of 0.93/88% and 0.92/90%. However, these combinations were not statistically superior to ADC alone.Discussion
The advantage of using advanced sequences instead of conventional T1- and T2-weighted imaging to evaluate the severity of fibrosis has been demonstrated in this radiation-induced model of colitis. The restriction of molecular diffusion and the vascular alterations induced by fibrosis were observed through a decrease in ADC. The presence of fibrotic tissue increased MTRnorm, as expected4. Finally, the hypovascular nature of fibrosis was also observed through decreased perfusion measurements.Conclusion
ADC, MTR and perfusion measurements were able to distinguish submucosal and transmural fibrosis in a murine model of radiation-induced colitis. This approach might be useful for diagnosis and assessment of anti-fibrotic treatments in patients.Acknowledgements
No acknowledgement found.References
1. Zappa M, Stefanescu C, Cazals-Hatem D et al. Which magnetic resonance imaging findings accurately evaluate inflammation in small bowel Crohn’s disease? A retrospective comparison with surgical pathologic analysis. Inflamm Bowel Dis. 2011;17(4):984-993.
2. Rieder F, Fiocchi C. Intestinal fibrosis in inflammatory bowel disease – current knowledge and future perspectives. J Crohns Colitis. 2008;2(4):279-290.
3. Tielbeek JA, Ziech ML, Li C et al. Evaluation of conventional, dynamic contrast-enhanced and diffusion weighted MRI for quantitative Crohn’s disease assessment with histopathology of surgical specimens. Eur Radiol. 2014;24(3):619-629.
4. Adler J, Swanson SD, Schmiedlin-Ren P et al. Magnetization transfer helps detect intestinal fibrosis in an animal model of Crohn disease. Radiology. 2011;259(1):127-135.
5. Jullien N, Blirando K, Milliat F et al. Upregulation of endothelin type a receptor in human and rat radiation proctitis: preclinical therapeutic approach with endothelin receptor blockade. Int J Radiat Oncol Biol Phys. 2009;74(2):528-538.
6. Erben U, Loddenkemper C, Doerfel K et al. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int J Clin Exp Pathol. 2014;7(8):4557-4576.
Figures