0828
Comparison of T2WI and DWI qualitative assessment and T2W-based radiomic features for predicting complete response in patients with rectal cancer after neoadjuvant chemoradiotherapy1Memorial Sloan Kettering Cancer Center, New York, NY, United States
Synopsis
Radiomics is a novel science that encompasses a computer-based extraction of quantitative features from images. Some studies have demonstrated that radiomics may help distinguishing malignant from benign diseases. We hypothesize that radiomics extracted from T2WI may improve qualitative MRI assessment in the evaluating of complete response in patient with locally advanced rectal cancer after neoadjuvant chemoradiotherapy.
INTRODUCTION
Neoadjuvant chemoradiotherapy (CRT) followed by total mesorectal excision is the standard treatment for patients with locally advanced rectal cancer (LARC). Approximately 25% of patients show complete response (CR) after CRT and non-operative approach has been emerging for those patients as an alternative to resection1,2. Thus, the assessment of tumor response after neoadjuvant CRT has become crucial, but still challenging. Radiomics is a novel science that encompasses a computer-based extraction of quantitative features from images. Some studies have demonstrated that radiomics may help distinguishing malignant from benign diseases3-7. The purpose of this study is to compare T2WI and DWI qualitative assessment and T2W-based radiomic features for predicting complete response in patients with LARC after neoadjuvant CRT.
METHODS
The Institutional Review Board approved this retrospective study of 327 patients with LARC who underwent neoadjuvant CRT followed by restaging MRI and surgery. Patients were excluded if they had recurrent or mucinous tumor, poor image quality, or surgery >3 months after MRI, which led to final study population of 114 patients. Two radiologists reviewed the T2WI and DWI sequences and qualitative classified the post-treated area as CR or PR in a consensus. For radiomics assessment, one radiologist segmented the volume of interest on high-resolution T2WI. Haralick texture features (energy, entropy, correlation, contrast, homogeneity), Gabor edge images at angles 0, 45, 90 and 135, and Haralick textures on Gabor features were computed8. Synthetic minority oversampling technique was used to balance the number of complete response and partial response, and random forest (RF) classifier was trained using repeated five-fold cross-validation to distinguish them9-10.RESULTS
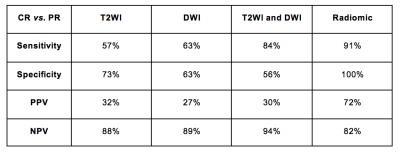
The sensitivity, specificity, PPV and NPV of T2WI, DWI and the combination of both and radiomics (RF classifier) for the diagnosis of CR are summarized in Figure 1. The combination of T2WI and DWI in differentiating CR from PR achieved a sensitivity of 84%, specificity of 56%, NPV of 94% and PPV of 30%. The RF classifier achieved an area under the curve (AUC) of 93% (95%CI; 89-96%) in differentiating CR from PR, with sensitivity, specificity, NPV and PPV of 91%, 100%, 72% and 82%, respectively.DISCUSSION
Overall, our results of qualitative assessment are in line with prior literature which report accuracy in the diagnosis of CR ranging from 50 to 90% among different groups and MRI sequences tested11-14. The combination of T2WI and DWI had higher sensitivity and NPV than previously described (84% and 94% vs 35% and 75%) and lower specificity and PPV (56% and 30% vs 94% and 75%)2. This difference can be justified due to variations in expertise among observers from different centers and due to variances in interpretation (5-point scale vs binary interpretation). With regards to the radiomics, our model had high accuracy, sensitivity and NPV in detecting CR, comparable to visual assessment. On the other hand, the RF classifier reached a much higher specificity and PPV when compared to qualitative evaluation. Considering that data augmentation was used, over-optimistic results may be achieved; however, cross-validation was used and this strategy has been used in some other medical databases15-18.
CONCLUSION
This preliminary study shows that T2W-based radiomic features may potentially improve the discrimination between complete and partial response. Although promising, these results require validation on independent dataset to assess the potential for clinical translation.Acknowledgements
No acknowledgement found.References
1. Maas M, Beets-Tan RG, Lambregts DM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(35):4633-40.
2. Maas M, Lambregts DM, Nelemans PJ, et al. Assessment of Clinical Complete Response After Chemoradiation for Rectal Cancer with Digital Rectal Examination, Endoscopy, and MRI: Selection for Organ-Saving Treatment. Annals of surgical oncology. 2015;22(12):3873-80.
3. Lubner MG, Stabo N, Abel EJ, Del Rio AM, Pickhardt PJ. CT Textural Analysis of Large Primary Renal Cell Carcinomas: Pretreatment Tumor Heterogeneity Correlates With Histologic Findings and Clinical Outcomes. AJR American journal of roentgenology. 2016;207(1):96-105.
4. Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology. 2016;278(2):563-77.
5. Sidhu HS, Benigno S, Ganeshan B, et al. "Textural analysis of multiparametric MRI detects transition zone prostate cancer". European radiology. 2017;27(6):2348-58.
6. Vargas HA, Veeraraghavan H, Micco M, et al. A novel representation of inter-site tumour heterogeneity from pre-treatment computed tomography textures classifies ovarian cancers by clinical outcome. European radiology. 2017.
7. Lakhman Y, Veeraraghavan H, Chaim J, et al. Differentiation of Uterine Leiomyosarcoma from Atypical Leiomyoma: Diagnostic Accuracy of Qualitative MR Imaging Features and Feasibility of Texture Analysis. European radiology. 2016.
8. Haralick RM SK, Dinstein IH. Textural features for image classification. Ieee T Syst Man Cyb: SMC, 1973; p. 610-21.
9. Breiman L. Random forests. Mach Learn. 2001;45(1):5-32.
10. Chawla NV, Bowyer KW, Hall LO, Kegelmeyer WP. SMOTE: Synthetic minority over-sampling technique. J Artif Intell Res. 2002;16:321-57.
11. Hotker AM, Tarlinton L, Mazaheri Y, et al. Multiparametric MRI in the assessment of response of rectal cancer to neoadjuvant chemoradiotherapy: A comparison of morphological, volumetric and functional MRI parameters. European radiology. 2016;26(12):4303-12.
12. Lambregts DM, Rao SX, Sassen S, et al. MRI and Diffusion-weighted MRI Volumetry for Identification of Complete Tumor Responders After Preoperative Chemoradiotherapy in Patients With Rectal Cancer: A Bi-institutional Validation Study. Annals of surgery. 2015;262(6):1034-9.
13. van der Paardt MP, Zagers MB, Beets-Tan RG, Stoker J, Bipat S. Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: a systematic review and meta-analysis. Radiology. 2013;269(1):101-12.
14. van den Broek JJ, van der Wolf FS, Lahaye MJ, et al. Accuracy of MRI in Restaging Locally Advanced Rectal Cancer After Preoperative Chemoradiation. Dis Colon Rectum. 2017;60(3):274-83.
15. Ueno Y, Forghani B, Forghani R, et al. Endometrial Carcinoma: MR Imaging-based Texture Model for Preoperative Risk Stratification-A Preliminary Analysis. Radiology. 2017:161950.
16. Corino VDA, Montin E, Messina A, et al. Radiomic analysis of soft tissues sarcomas can distinguish intermediate from high-grade lesions. Journal of magnetic resonance imaging : JMRI. 2017.
17. Zhang Y, Oikonomou A, Wong A, Haider MA, Khalvati F. Radiomics-based Prognosis Analysis for Non-Small Cell Lung Cancer. Sci Rep. 2017;7:46349.
18. Fehr D, Veeraraghavan H, Wibmer A, et al. Automatic classification of prostate cancer Gleason scores from multiparametric magnetic resonance images. Proc Natl Acad Sci U S A. 2015;112(46):E6265-73.