0823
MR elastography and multiparametric MRI of chronic pancreatitis reversal after bariatric surgery in obese rats1UMR 1149 Center For Research on Inflammation, Inserm, Paris, France, 2Beaujon Hospital, Pancreatology, AP-HP, Clichy, France, 3Beaujon Hospital, Pathology, AP-HP, Clichy, France, 4Bichat Hospital, Pathology, AP-HP, Paris, France, 5Beaujon Hospital, Radiology, AP-HP, Clichy, France
Synopsis
In obesity, pancreas is affected by fatty infiltration and fibrosis. Bariatric surgery is one of the only therapies which demonstrably improves pancreas status in obesity. In the present work we investigated the mechanical properties at several frequencies, the PDFF and the T2* of pancreatic explants in an obese rat model of bariatric surgery. Bariatric surgery reversed the MRI parameters to values not significantly different than controls. MRI parameters closely matched the reference histology observations. MRE and multiparametric MRI may be used to monitor pancreatic status and treatment response in an obese rat model of bariatric surgery.
Background
Obesity is a well-known risk factor for pancreatic diseases. Mechanisms involved include adipose tissue secreted pro-inflammatory cytokines, and insulin imbalance. In obese subjects, the pancreas contains fatty infiltration and fibrosis(1). We have previously shown that multiparametric MRI of pancreas explants combining the assessment of mechanical properties, fat fraction and T2*, is useful in estimating the pancreatic histopathology in a rat model of obesity(2). Bariatric surgery is one of the only treatments that can reverse diabetic symptoms(3). The effects of bariatric surgery on pancreatic status are, however, not well known. In this study, our aim was to analyze the effects of bariatric surgery on the MR imaging profile of the pancreas in an obese rat model.Methods
Animals: Three groups of male Wistar rats were composed: obese rats fed with high fat diet (HFD) for 6 months (n = 9); rats fed with normal diet (ND) for 6 months (n = 6) and obese rats which underwent bariatric surgery at 3 months, followed by ND for 14 days (n=5). After MR imaging, the pancreas were analyzed with hematoxylin and eosin staining for the quantification of fibrotic complexes and fat deposits, and Perls staining for the evaluation of iron deposition.
MRE: MRE was performed using a three-directional spin-echo sequence at 400Hz, 600Hz and 800Hz to accommodate the softness of pancreatic tissue(4), with 300µm isotropic resolution. Viscoelastic parameters (storage modulus, G' and loss modulus, G'' at all three frequencies, and their frequency dispersion coefficient, γ, expressed as the exponent of a fit to a power law) were obtained by algebraic inversion of the wave propagation equation.
R2* and proton density fat fraction (PDFF): acquisition consisted of a gradient echo sequence (TE=1.65ms+n·0.92ms, n=0···15) with 250µm in-plane resolution, 1mm slice thickness, 300kHz bandwidth, 950ms TR, 15° flip angle and 4 signal averages. T2* (expressed as R2*) and fat fraction were extracted by fitting the magnitude image to a model accounting for T2* decay and signal interferences from water and the 7 most abundant lipid peaks.
Results
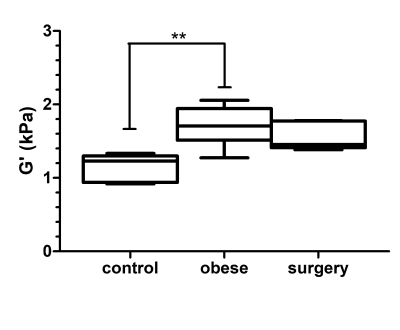
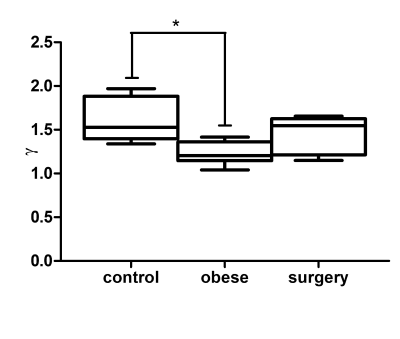
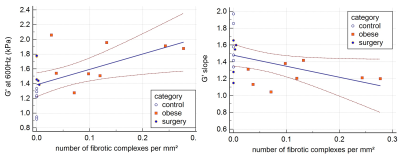
The storage modulus at 600Hz was significantly different between normal, obese and surgery groups (1.23[0.92-1.33]kPa, 1.71[1.27-2.06] kPa and 1.46[1.38-1.78] kPa, respectively, Kruskall-Wallis p=0.0037, figure 1). The storage modulus was significantly higher in obese rats versus controls (p < 0.01). After surgery the storage modulus decreased to a value that was not significantly different from controls. No significant differences were found between groups regarding the loss modulus at any of the three tested frequencies. The frequency dispersion coefficient of the storage modulus was significantly different between control, obese and surgical groups (1.53[1.34-1.97], 1.21[1.04-1.42] and 1.55[1.15-1.65], respectively, Kruskall-Wallis p=0.02, figure 2). After surgery the wave dispersion coefficient reverted to a value that was not significantly different from the control group. When pooling the measurements obtained from the three experimental groups, both the storage modulus G' at 600Hz and the storage modulus dispersion coefficient γG' were significantly correlated to the number of fibrotic complexes (r=0.61, p=0.0059 and r=-0.52, p=0.0216, respectively, figure 3).
The PDFF were 0.00[0.00-0.01]%, 0.39[0.00-1.59]%, and 0.20[0.00-0.49]% in the control, obese and surgical groups, respectively (Kruskal-Wallis p=0.014, figure 4), and were significantly correlated to the number of adipocytes per unit area (r = 0.71, p=0.0005). The R2* were 83.92[63.88-93.38] Hz, 104.39[94.14-164.42] Hz and 83.80[64.57-90.20] Hz for the control, obese and surgery groups, respectively (Kruskal-Wallis p=0.0008), and were significantly correlated to the number of Perls positive spots (figure 5).
Multiple regressions indicated that the number of endofibrotic complexes was the sole histologic factor associated to storage modulus at 600Hz while the frequency dispersion of the storage modulus was not associated to any single histologic factor. The number of adipocytes was the only histologic factor associated with PDFF, while the amount of Perls staining was the sole histologic factor associated with R2*.
Conclusions
Clinically accessible multiparametric MRI techniques could be used to assess the histological features of chronic pancreatitis in a rat model of obesity. The regression in histologic markers was accompanied with a reversion in the corresponding MRI quantitative markers of elastography, PDFF and R2*. This suggests that MRI markers including MR elastography may be used to assess not only pancreatic disease status but also to assess response to treatment after bariatric surgery.Acknowledgements
France Life Imaging network
Imageries Du Vivant network
References
1. Rebours V, Gaujoux S, d'Assignies G, et al. Obesity and Fatty Pancreatic Infiltration Are Risk Factors for Pancreatic Precancerous Lesions (PanIN). Clin Cancer Res. 2015;21(15):3522-8.
2. Garteiser P, Doblas S, Cavin J-B, et al. Abstract 1906: Pancreatic disease in obesity: observations on fat content, relaxometric properties and mechanical properties in the rat ex vivo. Powerpitch. Proceedings of the 2016 ISMRM conference. 2016.
3. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386(9997):964-73.
4. Shi Y, Glaser KJ, Venkatesh SK, Ben-Abraham EI, Ehman RL. Feasibility of using 3D MR elastography to determine pancreatic stiffness in healthy volunteers. Journal of magnetic resonance imaging: JMRI. 2015;41(2):369-75.
Figures