0822
Work dependence of calf muscle DTI exercise response from dynamic imaging1Bernard and Irene Schwartz Center for Biomedical Imaging, NYU Langone Medical Center, New York, NY, United States, 2Center for Advanced Imaging and Innovation (CAI2R), NYU Langone Medical Center, New York, NY, United States, 3Department of Radiology, NYU Langone Medical Center, New York, NY, United States
Synopsis
We describe measurement of work dependence of exercise response of diffusion contrast in calf muscle with a multiple echo diffusion tensor imaging (MEDITI) in a clinical 3 T scanner. With radial imaging, accelerated diffusion encoding, and compressed sensing reconstruction, spatial resolution of 3.7 mm and temporal resolution of 16 s was achieved. Using an MR-compatible ergometer with pneumatic resistance and force/displacement monitoring, post-exercise recovery of T2 and DTI metrics following plantarflexion in gastrocnemius muscle were monitored as a function of total normalized work. Exercise response showed significant anisotropy, and trends of higher response with work were observed.
Purpose
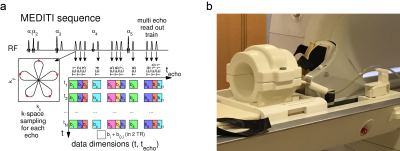
Diffusion tensor imaging (DTI) of skeletal muscle can be applied to probe myofiber architecture at rest, with extension, and in pathology. Exercise is a key challenge that has been widely employed with many MR contrasts1-4 including diffusion5-8. A method (MEDITATE) for compressing the required directional encodings has been demonstrated using multiple echoes9-11. A spatially-resolved variant (MEDITI) with a multi-spoke Single Trajectory Radial (STAR) k-space trajectory12 and compressed sensing reconstruction, has shown dynamic DTI of calf muscle13. A key aspect of MR exercise response is quantitative control and monitoring of exercise14-21. We evaluated MEDITI in calf muscle following in-scanner exercise in controls to probe the dependence of diffusion parameters on level of exertion. We used an MR-compatible ergometer with real time resistance control and performance monitoring.Methods
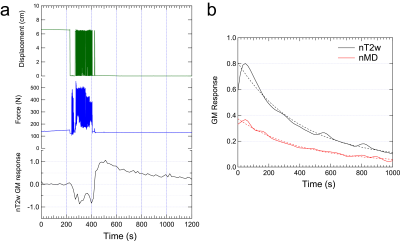
In this HIPAA-compliant, IRB-approved study, 7 normal controls (3 M , 4 F, ages 38 ±11 years) provided written informed consent for a unilateral calf MRI in a Siemens Skyra 3 T scanner and 15-channel Tx/Rx knee coil. Initially, subjects were positioned in an MR-compatible plantarflexion ergometer (Ergospect Trispect (Figure 1)) that provides real time control of pneumatic resistance and force / displacement measurement. The ergometer is vacuum-fixed to the table and the subject is connected to the ergometer via vest straps. Subjects performed an assessment of maximum voluntary contraction (MVC) against very high resistance. After 5 minute measured rest, subjects performed repeated plantarflexion for up to 3 minutes followed by 15 minute recovery. All subjects perfomed one bout (resistance = 27 ± 7 % MVC) and 3 subjects performed an additional bout of exercise (resistance = 40 ± 8 % MVC). Given force and displacement F(t) and x(t), normalized work was calculated as
\[{W_N} = \frac{1}{{MVC}}\int_0^T {F\left( t \right)\frac{{dx\left( t \right)}}{{dt}}} dt\]
where T is the duration of the exercise bout. MEDITI (Fig. 1a) captures 11 echoes with a 5-petal STAR-trajectory, with intershot angle increments according to the GRASP-scheme (Golden Radio Angle Radial Sparse Parallel). To maintain centering, field of view varied between subjects (FOV = 236+/-59 mm, resolution 3.7 ± 0.92 mm). Other parameters: TE: 90-245ms, isotropic b-values: 167-790 s/mm2, flip angles 61°/73°/85°/45°/85°, TR=2000ms, 10 mm slice thickness. Self-navigation corrected phase errors prior to dynamic reconstruction. Readouts from 4 TRs were combined for temporal resolution of 16 s. Sparsifying transforms exploited the similarity between diffusion weighted images along the echo (techo)- and the time (t)-dimensions to avoid undersampling artifacts22:
$$\hat X = \arg {\min _X}\left\{ {\left\| {E \cdot X - Y} \right\|_2^2 + {\lambda _{PCA}}{{\left\| {PC{A^{{t_{echo}}}}X} \right\|}_1} + {\lambda _{PCAt}}{{\left\| {PC{A^t}X} \right\|}_1} + {\lambda _{TV}}{{\left\| {T{V^{xy}}X} \right\|}_1}} \right\}$$
with X the time-series of images to be reconstructed, Y its k-space, E the multicoil encoding matrix, including coil sensitivities23 and the NuFFT-transform, PCA the Principal Component Analysis transform, TVxy the in plane total variation transform and λPCA, λPCAt and λTV regularization parameters. Time points during exercise were identified in a NuFFT reconstruction and edited out prior to final reconstruction. A cylindrical diffusion tensor model generated mean diffusivity (MD), axial (λ1), and radial (λrad) diffusion maps. Normalizing the post-exercise period by the average pre-exercise map generated diffusion response maps, which were temporally smoothed. Gastrocnemius medialis (GM) compartments were segmented on T2-weighted images and each recovery was fit to a monoexponential decay with baseline to estimate total response and decay rate. Response levels and decay rates were compared for all exercise bouts with Student’s t-tests, and Pearson correlations were measured between all response metrics and normalized work.
Results
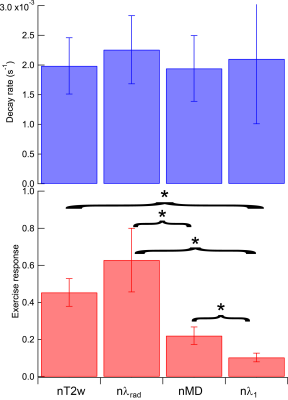
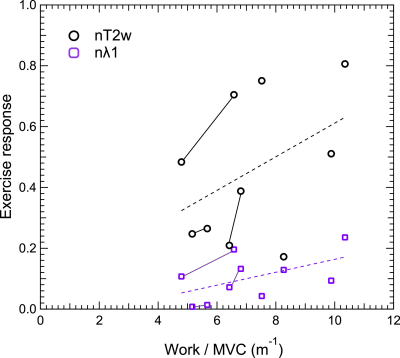
Example raw T2-weighted signal from NuFFT reconstruction along with force / displacement data (Fig. 2) shows response / recovery in GM, quantified by exponential decay in the full reconstruction. Spatial patterns of nT2w and nMD exercise response confirm GM activation (Figure 3). Group results (Figure 4) of exercise response levels show significant differences between all pairs of parameters (nT2w, nMD, nλrad, nλ1) except nT2w vs. nλrad. Decay rates for all parameters showed no group differences. nT2w (r = 0.44, p=0.20) and nλ1 (r=0.54, p=0.11) showed trends of association with normalized work (Figure 5).Discussion
This study shows feasibilty of dynamic DTI contrast in calf muscle with controlled / monitored exercise via MR-compatible pneumatic resistance ergometer. Anisotropy was observed in response levels but not in decay rates, with radial exceeding mean and axial diffusion response and T2-weighted response. Diffusion exercise response showed some association with higher normalized work in this pilot cohort. Some variance exists that further recruitment, subject training, and protocol uniformity may mitigate to study these trends more carefully and understand their diagnostic use.Acknowledgements
This work was supported by the NIH (R21EB009435).References
1. Zhou H, Novotny JE. Cine phase contrast MRI to measure continuum Lagrangian finite strain fields in contracting skeletal muscle. Journal of Magnetic Resonance Imaging 2007;25(1):175-184.
2. Litwiller D, Amrami K, Dahm D, Smith J, Laskowski E, Stuart M, Felmlee J. Chronic exertional compartment syndrome of the lower extremities: improved screening using a novel dual birdcage coil and in-scanner exercise protocol. Skeletal Radiology 2007;36(11):1067-1075.
3. Bendahan D, Giannesini B, Cozzone PJ. Functional investigations of exercising muscle: a noninvasive magnetic resonance spectroscopy-magnetic resonance imaging approach. Cellular and Molecular Life Sciences (CMLS) 2004;61(9):1001-1015.
4. Andreisek G, White LM, Sussman MS, Langer DL, Patel C, Su JWS, Haider MA, Stainsby JA. T2*-Weighted and Arterial Spin Labeling MRI of Calf Muscles in Healthy Volunteers and Patients With Chronic Exertional Compartment Syndrome: Preliminary Experience. Am J Roentgenol 2009;193(4):W327-W333.
5. Ababneh ZQ, Ababneh R, Maier SE, Winalski CS, Oshio K, Ababneh AM, Mulkern RV. On the correlation between T(2) and tissue diffusion coefficients in exercised muscle: quantitative measurements at 3T within the tibialis anterior. MAGMA 2008;21(4):273-278.
6. Morvan D, Leroy-Willig A, Malgouyres A, Cuenod CA, Jehenson P, Syrota A. Simultaneous temperature and regional blood volume measurements in human muscle using an MRI fast diffusion technique. Magn Reson Med 1993;29(3):371-377.
7. Filli L, Boss A, Wurnig MC, Kenkel D, Andreisek G, Guggenberger R. Dynamic intravoxel incoherent motion imaging of skeletal muscle at rest and after exercise. NMR Biomed 2015;28(2):240-246.
8. Rockel C, Akbari A, Kumbhare DA, Noseworthy MD. Dynamic DTI (dDTI) shows differing temporal activation patterns in post-exercise skeletal muscles. MAGMA 2016.
9. Baete SH, Cho G, Sigmund EE. Multiple-echo diffusion tensor acquisition technique (MEDITATE) on a 3T clinical scanner. NMR Biomed 2013;26(11):1471-1483.
10. Baete SH, Cho GY, Sigmund EE. Dynamic diffusion-tensor measurements in muscle tissue using the single-line multiple-echo diffusion-tensor acquisition technique at 3T. NMR Biomed 2015;28(6):667-678.
11. Sigmund EE, Baete SH, Luo T, Patel K, Bruno M, Mossa D, Stoffel D, Femia A, Ramachandran S, Franks A, Bencardino J. Assessment of thigh muscle in healthy controls and dermatomyositis patients with diffusion tensor imaging, intravoxel incoherent motion, and dynamical DTI. Proceedings of International Socieity of Magnetic Resonance in Medicine 2015; Toronto.
12. Sarty GE. Single TrAjectory radial (STAR) imaging. Magn Reson Med 2004;51(3):445-451.
13. Baete S, Raya JG, Knoll F, Cho GY, Parasoglou P, Brown R, Block KT, Otazo R, Bencardino J. Feasibility of In Vivo Dynamic Diffusion Tensor Imaging on a 3T clinical scanner with a Multi Echo Sequence and compressed sensing reconstruction. 2015; Toronto.
14. Gusso S, Salvador C, Hofman P, Cutfield W, Baldi JC, Taberner A, Nielsen P. Design and testing of an MRI-compatible cycle ergometer for non-invasive cardiac assessments during exercise. Biomedical engineering online 2012;11:13.
15. Tschiesche K, Rothamel M, Rzanny R, Gussew A, Hiepe P, Reichenbach JR. MR-compatible pedal ergometer for reproducible exercising of the human calf muscle. Medical engineering & physics 2014;36(7):933-937.
16. Weber TF, von Tengg-Kobligk H, Kopp-Schneider A, Ley-Zaporozhan J, Kauczor HU, Ley S. High-resolution phase-contrast MRI of aortic and pulmonary blood flow during rest and physical exercise using a MRI compatible bicycle ergometer. European journal of radiology 2011;80(1):103-108.
17. Pesta D, Paschke V, Hoppel F, Kobel C, Kremser C, Esterhammer R, Burtscher M, Kemp GJ, Schocke M. Different Metabolic Responses during Incremental Exercise Assessed by Localized 31P MRS in Sprint and Endurance Athletes and Untrained Individuals. Int J Sports Med 2013;34(08):669-675.
18. Esterhammer R, Schocke M, Gorny O, Posch L, Messner H, Jaschke W, Fraedrich G, Greiner A. Phosphocreatine kinetics in the calf muscle of patients with bilateral symptomatic peripheral arterial disease during exhaustive incremental exercise. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging 2008;10(1):30-39.
19. Kemp GJ, Meyerspeer M, Moser E. Absolute quantification of phosphorus metabolite concentrations in human muscle in vivo by 31P MRS: a quantitative review. NMR in biomedicine 2007;20(6):555-565.
20. Caterini JE, Elzibak AH, St Michel EJ, McCrindle BW, Redington AN, Thompson S, Noseworthy MD, Wells GD. Characterizing blood oxygen level-dependent (BOLD) response following in-magnet quadriceps exercise. MAGMA 2015;28(3):271-278.
21. Naimon ND, Walczyk J, Babb JS, Khegai O, Che X, Alon L, Regatte RR, Brown R, Parasoglou P. A low-cost Mr compatible ergometer to assess post-exercise phosphocreatine recovery kinetics. MAGMA 2017;30(3):281-289.
22. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med 2007;58(6):1182-1195.
23. Otazo R, Kim D, Axel L, Sodickson DK. Combination of compressed sensing and parallel imaging for highly accelerated first-pass cardiac perfusion MRI. Magn Reson Med 2010;64(3):767-776.
Figures