0811
Effect of lonidamine on response to temozolomide in a human glioma model1University of Pennsylvania, Philadelphia, PA, United States, 2Thomas Jefferson University, Philadelphia, PA, United States, 3Regina Elena National Cancer Institute, IRCCS, Rome, Italy, 4University of Alabama, Birmingham, AL, United States
Synopsis
The treatment of glioblastoma multiforme (GBM) includes temozolomide (TMZ) chemotherapy concurrent with radiotherapy. Lonidamine (LND), an inhibitor of monocarboxylate transporters 1&4, the mitochondrial pyruvate carrier, and complex II, is shown here to potentiate TMZ-induced growth inhibition of U251 glioma cells. Through LC-Mass Spectrometry of cells and 31P and 1H MRS of U251 xenografts, we identified mechanisms of this potentiation, including tumor-selective inhibition of bioenergetics (βNTP/Pi) and simultaneous acidification (intracellular pH and lactate) which may inhibit enzymes contributing to TMZ resistance such as glutathione-S-transferase and O6-methylguanine DNA methyltransferase (MGMT). LND may improve the current care of glioma patients and potentially overcome TMZ resistance.
INTRODUCTION
The Stupp protocol is the current standard of care for glioblastoma multiforme (GBM) patients.1 This protocol prescribes radiotherapy (RT) with concurrent and adjuvant temozolomide (TMZ) chemotherapy. The aim of this study was to use lonidamine (LND) to potentiate TMZ. LND inhibits the plasma membrane monocarboxylate transporters 1&4 (MCTs), the mitochondrial pyruvate carrier (MPC) and complex II of electron transport chain, inducing intracellular acidification, lactate accumulation, inhibition of ATP production and the oxygen consumption rate.2, 3 We anticipate that acidification, de-energization and tumor oxygenation will potentiate TMZ. In addition, we anticipate that selective tumor acidification will inhibit glutathione-S-transferase (GST) which neutralizes the diazomethane active intermediate of TMZ and O6-methylguanine methyltransferase (MGMT) that repairs DNA methylation.METHODS
U251 glioma cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine, and 1% penicillin-streptomycin. 2×106 U251 cells were inoculated subcutaneously (s.c.) in each mouse (n=5) as a 0.1 mL suspension.
31P and 1H MRS experiments (n=5) were performed after positioning the s.c. tumor in a dual-frequency slotted-tube resonator. The intracellular pH (pHi), extracellular pH (pHe), bioenergetics (βNTP/Pi), and lactate concentrations were measured after LND (100 mg/kg; i.p.) administration. Procedures for data acquisition, post-processing and parameter estimation were performed as previously described.4
U251 glioma cells were pretreated with DMSO or LND (150 μM) for 1 hour before treatment with DMSO or TMZ (300 μM) for 2 hours. Metabolites were extracted using methanol-water (4:1) and measured by LC-Mass Spectrometry. The metabolite levels were normalized with respect to controls.
In vitro growth inhibition studies were conducted in 6-well plates seeded with 1 × 105 U251 cells. Dose-response experiments for DMSO and LND showed their IC50s to be 1% and 150 µM, respectively. A subsequent dose-response experiment was performed for 72 h of TMZ treatment at various concentrations (0, 5, 25, and 75 µM) with DMSO or LND (150 µM, 1 hour pre-treatment). Cells were trypsinized and counted with trypan blue.
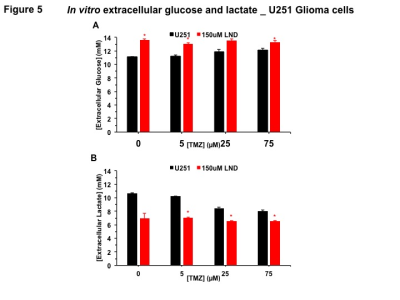
Extracellular glucose and lactate concentrations were measured using the YSI 2300 STAT Plus Glucose & Lactate Analyzer.
One-way ANOVA and t-test analyses were performed for statistical considerations
RESULTS
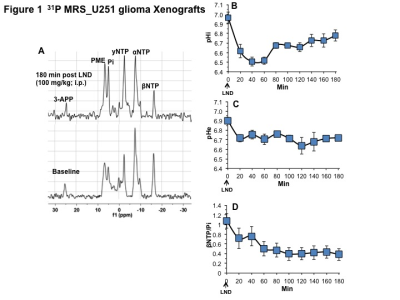
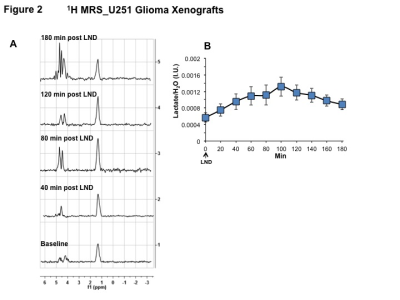
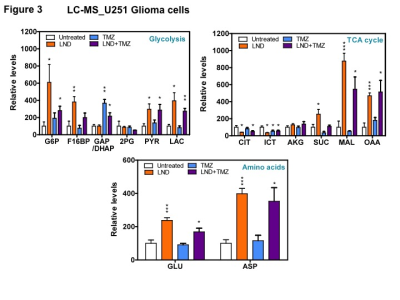
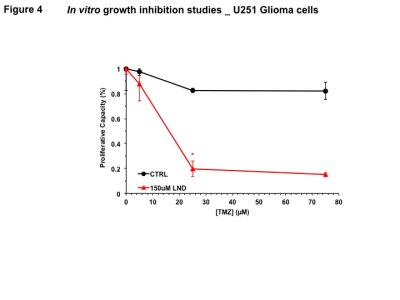
Representative localized 31P MR spectra of U251 glioma xenografts before and after LND treatment are shown in Figure 1A. LND produced a sustained and tumor-selective decrease in pHi from 6.95 ± 0.06 to 6.51 ± 0.04 (p < 0.001) (Fig. 1B), pHe from 6.91 ± 0.03 to 6.71 ± 0.03 (p > 0.05) (Fig. 1C). Tumor bioenergetics (βNTP/Pi) decreased by 73.0 ± 0.09% (p = 0.01) (Fig. 1D) relative to the baseline level immediately prior to LND administration. Steady-state levels of tumor lactate were monitored by 1H MRS with the HDMD-Sel-MQC transfer pulse sequence in U251 glioma s.c. xenografts after LND administration. The lactate intensity continuously increased until 100 min and then decreased monotonically (Figs 2 A & B). In vitro LC-MS studies demonstrated an altered metabolism of U251 with TMZ upon LND pre-treatment (Fig. 3). Compared to TMZ alone, LND+TMZ showed different levels of glycolytic and TCA cycle metabolites. Growth inhibition studies show that LND + TMZ decrease the proliferative capacity of U251 (Fig. 4). There is also increased glucose and decreased lactate in the extracellular medium of TMZ-treated U251 cells upon LND pre-treatment (Fig. 5).DISCUSSION
We expect that LND will induce tumor acidification and de-energization that will inhibit GST and MGMT activity and reduction of glutathione,5 which should increase ROS production which is essential to TMZ activity and block repair of TMZ damage to DNA. Also, inhibition of mitochondrial activity by LND will offset the effect of TMZ on the electron-transport chain, and decrease ATP, which should inhibit multi-drug resistance and raise TMZ levels in the tumor cells. In vitro studies demonstrate that LND potentiates TMZ in U251. One hour pre-treatment with 150 µM LND enhanced TMZ inhibition of tumor growth, even after accounting for growth inhibition effects of LND alone. LND may have a synergistic effect with TMZ, which we propose is due to the LND acidification effect which inactivates many key enzymes that produce TMZ resistance, including those responsible for DNA repair. Furthermore, LND pre-treatment inhibits respiration in U251 cells, thereby increasing oxygenation of tumors to potentiate TMZ chemotherapy.CONCLUSIONS
This study seeks to improve treatment of glioma patients by overcoming TMZ resistance. LND may attenuate resistance demonstrated by high levels of MGMT enzyme, which is critical to DNA repair of TMZ-induced damage. RT may also be potentiated by LND through the inhibition of respiration and subsequent increased ROS generation.Acknowledgements
NIH grants R01-CA129544 and R01-CA172820References
1. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-66.
2. Guo LL, Shestov AA, Worth AJ, et al. Inhibition of Mitochondrial Complex II by the Anticancer Agent Lonidamine. Journal of Biological Chemistry. 2016;291(1):42-57.
3. Nancolas B, Guo LL, Zhou R, et al. The anti-tumour agent lonidamine is a potent inhibitor of the mitochondrial pyruvate carrier and plasma membrane monocarboxylate transporters. Biochemical Journal. 2016;473:929-36.
4. Nath K, Nelson DS, Ho AM, et al. 31P and 1H MRS of DB-1 melanoma xenografts: lonidamine selectively decreases tumor intracellular pH and energy status and sensitizes tumors to melphalan. NMR Biomed. 2013;26(1):98-105. PMCID: 3465621.
5. Rocha CR, Garcia CC, Vieira DB, et al. Glutathione depletion sensitizes cisplatin- and temozolomide-resistant glioma cells in vitro and in vivo. Cell Death Dis. 2014;5:e1505.
Figures