0805
Dynamic contrast enhanced MRI detects pancreatic cancer responses to stroma-directed therapy1Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Pancreatic Cancer Research Center, University of Pennsylvania, Philadelphia, PA, United States, 3Abramson Cancer Center, University of Pennsylvania, philadelphia, PA, United States, 4Halozyme Therapeutics, San Diego, CA, United States
Synopsis
Stroma-targeted
Introduction
Pancreatic ductal adenocarcinoma (PDA) is a leading cause of cancer death hence there is a race to identify effective therapies for this deadly cancer. PDA is characterized by a dense stroma, which is populated by non-cancer cells and extracellular matrix. The stroma presents a microenvironment that is immune-suppressive and poses a substantial barrier to penetration of chemotherapeutic drugs. Overcoming this barrier appears to be essential for successful management of PDA in the clinic. While stroma-directed drugs are being examined in the preclinical and clinical phases, quantitative imaging markers are not available to evaluate various stroma-directed approaches. In this study, we hypothesized that DCE-MRI can be employed to detect stromal changes. DCE-MRI was performed before and after treatment with the stroma-directed investigational drug, PEGPH20, in subcutaneous(subQ), orthotopic models and genetically engineered murine model (GEMM) of PDA.Method
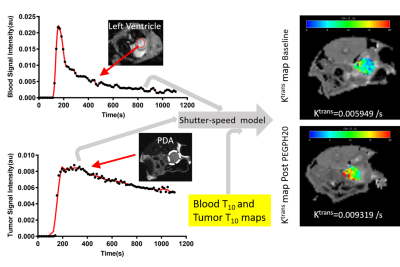
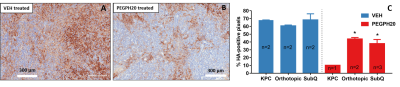
PEGPH20 (Halozyme Therapeutics) is a human recombinant hyaluronidase that degrades a stroma component, hyaluronan (HA). SubQ xenografts of human PDA were generated in athymic mice by flank injection of 10-million BxPC-3 cells (ATCC). Orthotopic model was produced by injection of 1.25x105 murine PDA cells 1 into the pancreas of C57BL/6 mice. GEMM harboring a pancreas specific Cre allele and p53 and Kras mutation (KPC) 2 is maintained at our institute. SubQ and orthotopic models were scanned at baseline and 24 h post one intravenous (iv) injection of PEGPH20 (1 mg/kg) or vehicle (VEH). KPC mice received an injection of PEGPH20 or VEH on day 0, 7, and 14 was followed by iv injection of gemcitabine (50 mg/kg) on the consecutive day. MRI was performed in KPC mice before the treatment was started and then one day after it was completed. MRI studies were performed on 9.4T system (Agilent) while under isoflurane anesthesia the mouse was placed inside a 35-mm ID x 10 cm long quadrature birdcage transceiver coil (M2M). The DCE-MRI protocol includes T10 mapping and DCE series using an ECG-gated multislice saturation-recovery gradient-echo sequence with Cartesian k-space sampling 3, 4. A 0.2 mL bolus of MultiHance (Bracco) diluted 20 mM in saline was injected via tail vein catheter using a syringe pump (Harvard Apparatus). A micro controller based device was used to record pulse sequence timing of the ECG-gated acquisition to correct timing errors due to irregularities in the ECG triggering. The corrected dynamic images, T10 map and AIF data were then fit to the Shutter-Speed model 3, 5 using least squares methods to yield maps of Ktrans, Ve and τi (Fig 1). Immunostaining for HA was performed on tumor sections 6 and the fraction of HA-positive pixels was estimated (Leica).Result
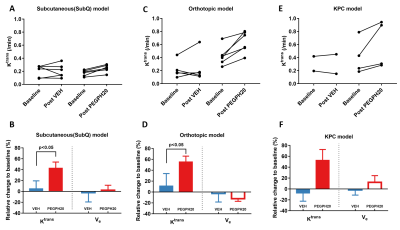
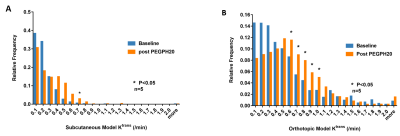
Paired imaging studies (pre and post treatment) clearly show an increase of Ktrans in all mice treated by PEGPH20 (Fig 2A, C) or PEGPH20 plus gemcitabine (Fig 2E). Change of Ktrans before and after treatment revealed a significant increase as early as 24 h after one injection of PEGPH20 in the subQ and orthotopic models (Fig 2B, D). The efficacy of PEGPH20 in depleting HA content in tumor extracellular matrix was confirmed by immunostaining (Fig 3A-C). Histograms of Ktrans (Fig 4A-B) showed a broader distribution in the orthotopic model relative to subQ model. Meanwhile, the range of baseline Ktrans values is similar between KPC vs. orthotopic models (Fig 2 C and E). After multiple stromal plus chemo drug treatments, an increase in Ktrans was observed in KPC mice, suggesting that the combined treatment led to normalized perfusion rather than vascular shutdown (Fig 2E-F). Neither blood nor tumor T10 were sensitive to the treatment. An excellent inter-animal reproducibility of the T10 was obtained (Fig 5A-C).Discussion
Although DCE-MRI has been extensively applied to characterize responses to the anti-vascular or anti-angiogenesis therapy 7, its application in stroma-directed therapy has not been examined. Our results suggest that this technique can detect stromal changes as early as 24 h post treatment, and the MRI results are corroborated with HA-staining. The increased vascular permeability/ perfusion measured by Ktrans is consistent with prior reports that PEGPH20 treatment reduced tumor interstitial fluid pressure, leading to re-opening of the otherwise collapsed capillaries 8. The stroma-directed therapy in combination with gemcitabine induce an increase in Ktrans, therefore, the current application would have a favorable dynamic range and sensitivity. Conclusion
These results show the utility of DCE-MRI in monitoring the changes of tumor microenvironment induced by stroma-directed drugs in murine PDA models. Future studies can be designed to test the utility of the quantitative imaging technique in PDA patients receiving PEGPH20 and in combination with chemotherapy.Acknowledgements
We thank the support of the Mouse Hospital of Penn Pancreatic Cancer Research Center. This study was partially supported by the Institute for Translational Medicine and Therapeutics (ITMAT) and by the National Center for Research Resources, Grant UL1RR024134, which is now at the National Center for Advancing Translational Sciences, Grant UL1TR000003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
1. Lo, A., L.C. Wang, J. Scholler, et al., Tumor-Promoting Desmoplasia Is Disrupted by Depleting FAP-Expressing Stromal Cells. Cancer Res, 2015. 75(14): p. 2800-10.
2. Hingorani, S.R., E.F. Petricoin, A. Maitra, et al., Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell, 2003. 4(6): p. 437-50.
3. Zhou, R., S. Pickup, T.E. Yankeelov, et al., Simultaneous measurement of arterial input function and tumor pharmacokinetics in mice by dynamic contrast enhanced imaging: effects of transcytolemmal water exchange. Magn Reson Med, 2004. 52(2): p. 248-57.
4. Pickup, S., R. Zhou, and J.D. Glickson, MRI estimation of the arterial input function in mice. Academic Radiology, 2003. 10: p. 963-968.
5. Li, X., W.D. Rooney, and C.S. Springer, Jr., A unified magnetic resonance imaging pharmacokinetic theory: intravascular and extracellular contrast reagents. Magn Reson Med, 2005. 54(6): p. 1351-1359.
6. Jadin, L., L. Huang, G. Wei, et al., Characterization of a novel recombinant hyaluronan binding protein for tissue hyaluronan detection. Journal of Histochemistry & Cytochemistry, 2014. 62(9): p. 672-683.
7. O'Connor, J.P., A. Jackson, G.J. Parker, et al., Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat Rev Clin Oncol, 2012. 9(3): p. 167-77.
8. Provenzano, P.P., C. Cuevas, A.E. Chang, et al., Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell, 2012. 21(3): p. 418-29.
Figures