0802
Crosstalk of atrophic trigeminal nerve to abnormal brain structure in idiopathic trigeminal neuralgia1Department of Medical Imaging, First affiliated hospital of Xi'an Jiaotong University, Xi'an, China, 2Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3Department of Neural and Pain Sciences, School of Dentistry, University of Maryland Baltimore, Baltimore, MD, United States
Synopsis
Idiopathic trigeminal neuralgia (ITN) is characterized by intermittent, lancinating attacks in the branches of the trigeminal nerve (TGN). 40 ITN patients and 40 matched controls underwent MRI sessions and clinical pain assessment. TGN volume of cisternal segment and whole brain grey matter volume (GMV) were evaluated using voxel-based morphometry, ect. Reduced GMV was found in the insula, dorsal anterior cingulate cortex, precuneus, and several areas of the temporal lobe in ITN subjects. Correlation analysis revealed that decreased GMV of the left insula and decreased TGN volume were associated with increased pain ratings, providing new insight into pathophysiology of the disease.
Introduction
Recent neuroimaging studies have reported grey
matter (GM) alterations in idiopathic trigeminal neuralgia (ITN) patients.
However, few studies have focused on quantitative measurements of trigeminal
nerves (TGN) and the interaction between TGN volume and
brain morphology, particularly GM volume (GMV). In this study, we investigated the
link between TGN and GMV changes in ITN patients compared to healthy controls. Moreover,
we explored the association of microstructure of TGN and GM to collected pain clinical
variables.Methods
Eighty participants (40 ITN patients and 40
matched healthy controls, Table 1) were recruited for the study. All
participants
underwent magnetic resonance imaging sessions and clinical pain
assessment. TGN
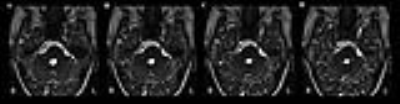
volume of cistern segment was measured by Medical Image
Processing, Analysis, and Visualization software (MIPAV,
http://mipav.cit.nih.gov, Figure 1), and whole brain GMV was evaluated
using voxel-based morphometry implemented in SPM12. Sensory and
affective pain
rating indices were assessed using visual analog
scale (VAS) and the short-form McGill Pain Questionnaire (SF-MPQ).
Correlation analysis
was conducted to investigate the relationship between clinical pain
variables
and volumetric changes in TGN and GM.Results
The average volume of the affected TGN compared to the unaffected TGN in ITN patients was significantly smaller. This was calculated to be an average volumetric reduction of 22.8% from the unaffected to affected TGN. Meanwhile, the affected TGN of ITN patients was statistically smaller than the average volume of bilateral TGNs in the control subjects (Figure 2A). Additionally, the volume of the affected TGN was negatively correlated with the VAS scores (Figure 2B) and total, affective, and sensory pain rating indices of SF-MPQ (Figure 2C) in ITN patients.
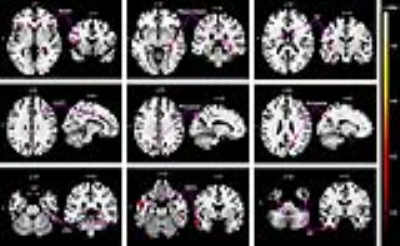
What's more, reduced GMV was found in several regions associated with pain in ITN subjects, including the insula, secondary somatosensory cortex, hippocampus, dorsal anterior cingulate cortex, precuneus, and several areas of the temporal lobe (Figure 3 and Table 2). In addition, a positive correlation was found between the GMV of the left insula and the volume of affected TGN in the patients (not shown).
Discussion
Previous MRI studies mainly focused on nerve vessel contact (NVC) of affected TGN in ITN patients, but NVC is frequently observed in unaffected TGN in patients and controls, demonstrating that qualitative assessment of NVC is not enough to act as a diagnostic component for ITN. Atrophy rarely occurs on the unaffected TGN in ITN patients or on bilateral TGNs in healthy subjects, however, it is prevalent on the affected TGN of ITN individuals and is undoubtedly a potential factor in the underlying etiology of ITN.
Except popular impared brain regions, our results revealed GMV atrophy in the bilateral temporal pole, right inferior temporal gyrus (ITG), and left middle temporal gyrus (MTG). Although decreased GMV of the temporal lobe is not commonly detected in chronic pain states, it has been observed more frequently in ITN patients. A possible interpretation is that anticipation of the severe pain recruits the limbic system including hippocampus, temporal pole, and ITG to modulate aversive memory recognition, which, in turn, support the involvement of the temporal lobe in the anticipation of sporadic and lancinating orofacial pain.
Our data suggests that the microstructure of the affected TGN may present as an important factor in predicting the development and severity of ITN. But up until now there was little focus on ITN in conjunction with TGN microstructure deficits and brain morphological alterations. Future studies should focus on morphometric abnormalities of the affected TGN and whole brain in mild cases of ITN in order to observe changes in GMV as the disease progresses.
Conclusion
Our study observed
structural deficits of affected TGN and brain GM atrophy in ITN patients. We
not only demonstrate a correlation of the affected TGN to the left insula GMV,
indicating a potential link between the cranial nerve and cerebral cortex, but
we show that volumetric measurement of TGN is associated with multiple pain dimensions
and pain severity in ITN patients. Above all, these findings complement the
microstructural investigations of TGN and whole brain GMV changes in ITN
patients, which help to provide greater clarity to understanding its underlying
pathophysiology.Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (No. 81301207), the Clinical Research Award of the First Affiliated Hospital of Xi’an Jiaotong University, China (No. XJTU1AF-CRF-2015-028), and the Opening Project of Key Laboratory of Shaanxi Province for Craniofacial Precision Medicine Research, College of Stomatology, Xi’an Jiaotong University (No. 2016LHM-KFKT001). Dr. Ming Zhang and Dr. David Seminowicz are joint corresponding authorsReferences
1. Wang Y, Li D, Bao F, et al. Microstructural abnormalities of the trigeminal nerve correlate with pain severity and concomitant emotional dysfunctions in idiopathic trigeminal neuralgia: A randomized, prospective, double-blind study. Magnetic resonance imaging, 2016, 34 (5): 609-616.
2. Leal PRL, Barbier C, Hermier M, et al. Atrophic changes in the trigeminal nerves of patients with trigeminal neuralgia due to neurovascular compression and their association with the severity of compression and clinical outcomes: Clinical article. Journal of neurosurgery, 2014, 120 (6): 1484-1495.
3. Obermann M, Rodriguez-Raecke R, Naegel S, et al. Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. NeuroImage, 2013, 74: 352-358.
4. Gustin SM, Peck CC, Wilcox SL, et al. Different Pain,
Different Brain: Thalamic Anatomy in Neuropathic and Non-Neuropathic Chronic
Pain Syndromes. Journal of Neuroscience, 2011, 31 (16): 5956-5964.
Figures