0778
Tropoelastin: A novel marker for atherosclerotic plaque instability1Biomedical Engineering, King's College London, London, United Kingdom, 2Centre de Biophysique Moléculaire, CNRS, Orleans, France, 3Radiology, Pontificia Universidad Católica de Chile, Santiago, Chile, 4Academic Department of Vascular Surgery, King's College London, London, United Kingdom
Synopsis
Elastolysis and ineffective
Introduction:
Elastin is an abundant protein of the arterial wall that contributes to 50% of its dry weight. Elastolysis and ineffective elastogenesis favour the accumulation of tropoelastin, rather than cross-linked elastin, in atherosclerotic plaques and has been associated with lesion progression and destabilization [1, 2]. Here, we employed in vivo MRI in a rabbit model of atherothrombosis to investigate the merits of a newly developed tropoelastin-binding gadolinium-based contrast agent (Gd-TESMA) for the detection of rupture-prone plaques. The elastin-binding contrast agent (Gd-ESMA) that binds to both cross-linked elastin and tropoelastin was used for comparison.Materials and Methods:
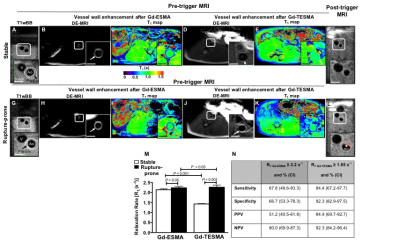
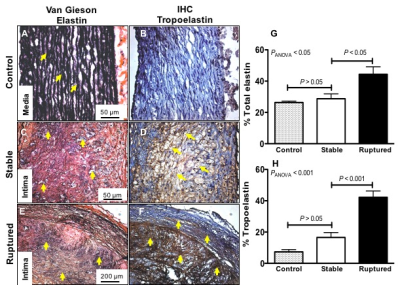
Animal model: Aortic atherosclerosis was induced in New Zealand White rabbits (n=6) by cholesterolemia and endothelial denudation. Plaque rupture and thrombosis was induced with Russell’s viper venom (0.15 mg/kg) and histamine (0.02 mg/kg) [3]. In vivo MRI: The abdominal aorta was scanned using a 3.0 T Philips Intera Scanner and a 32-channel cardiac coil. Rabbits were imaged twice before (pre) and one time after (post) pharmacological triggering. The pre-trigger MRI included two separate scanning sessions; before and after administration of Gd-ESMA (LMI1174) (Lantheus Medical Imaging, Billerica, MA) and another one before and after administration of Gd-TESMA. The second MRI was performed two days after the first to allow blood clearance of Gd-ESMA. During these sessions, native zoom T1BB and delayed-enhanced (DE) images and T1 maps were acquired 2 hours after administration of Gd-ESMA (0.2 mmol/kg) and 40min after administration of Gd-TESMA (0.2 mmol/kg), which were found to be the optimal time points. After the Gd-TESMA scan, the rabbits were triggered for plaque rupture and the final post-triggere MRI was performed 8h later. The post-trigger MRI included acquisition of native zoom T1BB images to visualize thrombus and classify plaques in ruptured and stable. ECG triggered T1BB images were acquired with: TR=2 heartbeats; TE=9.8ms profile order of low-high; echo train length=6, inversion delay of 350ms, FOV=60×17×104mm, matrix=200×54, resolution=0.3×0.3mm, slice thickness=4mm, slices=26. A two-dimensional Look-Locker sequence was used to determine the inversion time for blood signal nulling. Non-ECG gated 3D late gadolinium enhancement (LGE) inversion-recovery gradient-echo axial images were acquired with: TR/TE=7.1/2.8ms, flip angle =30°, FOV=120×85×100mm, matrix=520×369, resolution=0.25×0.25mm, slice thickness=4mm, slices=33, repetition time between subsequent inversion-recovery pulses, 1000ms. T1 mapping was performed using a 3D Modified Look-Locker Inversion Recovery (MOLLI) with segmented k-space acquisition that allows acquisition of T1 maps at a higher spatial resolution crucial for vessel wall mapping (4). The 3D MOLLI sequence employs two acquisition trains each preceded by a nonselective inversion pulse with inversion times ranging from 20 to 2000ms, followed by eight segmented readouts for eight individual images. T1 mapping parameters were: TR/TE=8.1/4.1ms, flip angle=35°, FOV=110×70×100, matrix=275×175, resolution=0.4×0.4mm, slice thickness=4mm, slices=25. The T1 maps were automatically generated on the reconstruction computer after the data acquisition using a 3-parameter fit model. The T1 maps were imported in Osirix and the vessel wall was manually segmented to calculate the T1/R1 values. Histology: Van Gieson elastin staining and tropoelastin immunohistochemistry were used ex vivo for validation.Results and Discussion:
Representative pre-trigger T1BB images of a stable (Fig. 1A-1E) and rupture-prone plaque (Fig. 1G-1K) show the location of the lesion. The LGE images show enhancement of the vessel wall and shortening of the T1 relaxation time both after the administration of Gd-ESMA and after the administration of Gd-TESMA. The post-trigger T1BB images show no thrombus at the location of the stable plaque and the presence of thrombus at the location of the rupture-prone lesion (Fig. 1F and 1L). Although both Gd-ESMA and Gd-TESMA resulted in vessel wall enhancement, quantitative assessment of the R1 relaxation rate showed that rupture-prone plaques uptake significantly more Gd-TESMA compared with stable plaques (R1=2.26±0.1 vs R1=1.43±0.02, P<0.001) whereas uptake of Gd-ESMA was similar between rupture-prone and stable plaques (R1=2.44±0.07 vs R1=2.14±0.05, P>0.05) (Fig. 1M). Gd-TESMA MRI was superior Gd-ESMA in detecting the rupture-prone plaque (Fig. 1N). Quantification of total elastin (Fig. 2A, 2C, 2E) and tropoelastin (Fig. 2B, 2D, 2F) as measured histologically showed that tropoelastin accumulation is significantly higher in ruptured compared to stable plaques (42.2±4.0 vs 16.6±3.14, P<0.001 while the total increase of elastin (28.8±3.0 vs 44.35±4.83 %, P<0.001), that includes both cross-linked mature and immature tropoelastin fibers, is similar between the two plaque types (Figure 2G-2H). Only negligible amounts of tropoelastin (7.3±1.45%) were found in control vessel wall compared with mature elastin (26.3±0.9%).
Conclusions:
Dysfunctional elastin turnover and accumulation of tropoelastin in the vessel wall is associated with lesion instability and thus may be a new biomarker for unstable plaque. Using a newly developed tropoelastin-binding gadolinium-based contrast we demonstrate, for the first time, that molecular imaging of tropoelastin allows discrimination of stable from rupture-prone plaques.Acknowledgements
The British Heart Foundation, the BHF Centre of Excellence, the Chilean Agency of Technology and Science and the Wellcome EPSRC Centre for Medical Engineering at King’s College London.References
1. Krettek, A., G.K. Sukhova, and P. Libby, Elastogenesis in human arterial disease: a role for macrophages in disordered elastin synthesis. Arterioscler Thromb Vasc Biol, 2003. 23(4): p. 582-7.
2. Makowski, M.R., et al., Assessment of atherosclerotic plaque burden with an elastin-specific magnetic resonance contrast agent. Nature medicine, 2011. 17(3): p. 383-8.
3. Phinikaridou, A., et al., In vivo detection of vulnerable atherosclerotic plaque by MRI in a rabbit model. Circ Cardiovasc Imaging. 3(3): p. 323-32.
4. Nezafat M, Henningsson M, Stehning C, Akcakaya M, Protti A, Botnar R. A segmented modified look-locker inversion recovery (MOLLI) sequence for high heart rate T1 mapping of mice. J Cardiovasc Mag Reson. 201517(Suppl 1):P120.