0771
Strain Measurements from 3D Isotropic Cine MRI: Relation with Fibrosis in a Duchenne Patient Population1IADI, INSERM U947 & Université de Lorraine, Nancy, France, 2CIC-IT 1433, INSERM, Université de Lorraine and CHRU Nancy, Nancy, France, 3Compter Science Department, Technical University Munich, Munich, Germany, 4Department of Cardiothoracic Surgery, CHU Strasbourg and University of Strasbourg, Strasbourg, France
Synopsis
A method is proposed for myocardial strain estimation, based on 3D isotropic cine MRI. A free-breathing, motion-corrected super-resolution acquisition is used to obtain the 3D isotropic cine dataset. Then the left ventricular (LV) myocardium is manually segmented using a sparse segmentation technique. Finally a registration-based technique is used to obtain displacement fields that best match diastolic and systolic 3D isotropic masks of the LV, and subsequent Lagrangian strain estimates. The method was evaluated in 20 Duchenne muscular dystrophy patients. Segment-wise analysis showed that the proposed radial strain estimates correlated well with the presence of fibrosis.
Introduction
Isotropic 3D cine images with high spatial and temporal resolution are becoming available in MRI thanks to advances in scan acceleration and/or super-resolution techniques1. This allows novel post-processing tools to be investigated. In this work we propose a workflow for: (i) acquisition and reconstruction of 3D isotropic cine data; (ii) sparse manual segmentation of the left ventricular (LV) myocardium; (iii) registration-based 3D strain estimation. The method was evaluated in a Duchenne patient population. Segment-wise analysis was performed to investigate the relation between radial strain estimates and the presence of fibrosis.Methods
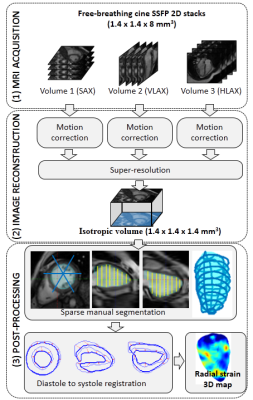
Patients: 20 patients with Duchenne muscular dystrophy (DMD) were included in this study. Their age was 12.9 ± 4.3 years old (minimum: 6; maximum: 20). Due to their breathing difficulties the whole examination was performed under free breathing with GRICS motion correction2,3. A 1.5T scanner was used (Signa HDxt, General Electric, Milwaukee, USA) with 8-channel cardiac coil. The processing workflow is illustrated in Fig. 1.
Imaging: The protocol included cine sequences (bSSFP) for functional assessment, with 2D multislice coverage of the left ventricle (LV) in short axis (SAX), horizontal and vertical long-axis (HLAX, VLAX). These three cine stacks (1.4x1.4x8 mm3 resolution) were motion-corrected and then combined into an isotropic (1.4x1.4x1.4 mm3) cine dataset, using super-resolution as described in Ref. 1. Tissue characterization sequences were performed after contrast agent injection, including 2D cine sequences (5 bSSFP slices), late gadolinium enhancement (LGE) after 10-15 min (3D IR-FGRE: SAX, HLAX, VLAX), and T1 mapping (1 slice at t=0, 1, 10, 15-20 min). For the LGE sequence, an additional breath-hold sequence was asked when possible, and GRICS motion correction was applied otherwise.
Strain calculation: Post-processing of the 3D isotropic cine data included a manual sparse segmentation of the LV. Several strategies were investigated to segment endocardium and epicardium (in diastole and systole): conventional SAX segmentation, or LV reconstruction from various combinations of reformatted SAX and LAX slices (in particular: 3 SAX + 3 LAX slices rotated by 60° about the longitudinal axis). Non-rigid 3D registration4 of the LV masks from diastole to systole was used to obtain estimates of the displacement fields and subsequent Lagrangian strain estimates. Strain values in the 17 AHA segments were obtained by averaging strain values from all voxels of the corresponding segments in the 3D isotropic strain map. Fibrosis assessment: Fibrosis was assessed qualitatively by an experienced cardiologist. When LGE was of poor image quality, post-contrast cine and T1 maps were also used. The location of fibrosis was reported (i.e. segment number). Two patient sub-groups were formed: group 1: no detectable fibrosis; group 2: evidence of fibrosis in one segment or more.
Results
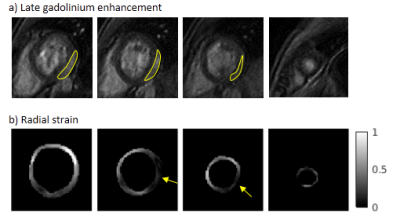
Isotropic 3D cine data and subsequent 3D strain maps were successfully reconstructed for all patients. Regarding fibrosis assessment, 2 patients were excluded (no accessible vein for injection for 1 patient; no interpretable fibrosis image for 1 patient). No evidence of fibrosis was found in 9 patients, and fibrosis was detected in the remaining 9 patients, mainly in inferolateral and inferior segments (see example in Fig. 2): AHA #11 (9 patients), #5 (7), #6 (4), #12 (3) etc...
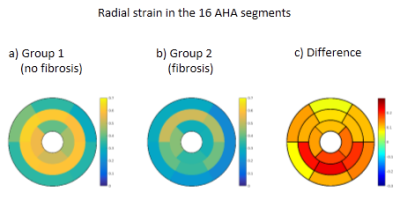
Radial strain was lower in all segments for group 2 (with fibrosis) compared to group 1, as can be seen in Fig. 3. The largest decrease of radial strain was observed in the medial segments #9, #10, #11 (0.36-0.41 VS 0.59-0.61), and in the basal segment #5 (0.19 VS 0.33).
Discussion and Conclusion
Unlike tagging MRI or myocardial feature tracking, the proposed method does not attempt to track the motion of local structures, but rather searches for the displacement field that best matches the global 3D geometry of the LV in diastole and systole. Here the displacement with minimal “spatial variation” energy was determined (minimal L2 norm of the spatial gradient). Actual biomechanical constraints (e.g. enforcing incompressibility) might improve the soundness of the registration results.
Nevertheless the segmental radial strain values were consistent with the identified fibrosis segments. The results also seem consistent with the literature on DMD, in terms of loss of local contractile function5 and distribution of fibrosis6. Only the results using the conventional, full SAX stack segmentation are presented here, but in future work we will investigate what is the minimal number of sparse reformatted slices that is needed for LV segmentation to obtain accurate radial strain estimates.
Acknowledgements
The patient study (ID C13-04) was funded by AFM (French association against myopathy). The authors also received support from the European Commission through Grant Number 605162.References
1. Odille F et al. Isotropic 3D cardiac cine MRI allows efficient sparse segmentation strategies based on 3D surface reconstruction. Magn Reson Med. 2017. In press. doi:10.1002/mrm.26923
2. Odille F, Vuissoz P-A, Marie P-Y & Felblinger J. Generalized reconstruction by inversion of coupled systems (GRICS) applied to free-breathing MRI. Magn Reson Med. 2008;60:146–157.
3. Bonnemains L. et al. Free-breathing with motion-correction and video projection during cardiac MRI : a paediatric design ! J. Cardiovasc Magn Reson. 2014;16:P319.
4. Odille F, Escanyé J-M, Atkinson D, Bonnemains L & Felblinger J. Nonrigid registration improves MRI T2 quantification in heart transplant patient follow-up. J Magn Reson Imaging. 2015;42:168–174.
5. Hor K N et al. Circumferential Strain Analysis Identifies Strata of Cardiomyopathy in Duchenne Muscular Dystrophy: A Cardiac Magnetic Resonance Tagging Study. J Am Coll Cardiol. 2009;53:1204–1210.
6. Hor K N et al. Prevalence and distribution of late gadolinium enhancement in a large population of patients with Duchenne muscular dystrophy: effect of age and left ventricular systolic function. J Cardiovasc Magn Reson. 2013;15:107.
Figures