0764
Quantitative myocardial perfusion using multi-echo Dixon for respiratory motion correction and arterial input function estimation1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Federal University of Minas Gerais, Belo Horizonte, Brazil, 3Federal Center for Technological Education of Minas Gerais, Belo Horizonte, Brazil, 4Philips Healt Systems, London, United Kingdom
Synopsis
A quantitative myocardial first-pass perfusion approach is proposed using multi-echo Dixon acquisition. The fat images (unaffected by contrast bolus) are used to estimate respiratory motion and the transformations are applied to the corresponding diagnostic water images. The three echo images are also used to perform T2* correction of the arterial input function. Evaluation was performed in 8 patients during free-breathing rest perfusion. Motion correction reduced quantiation variability, while T2* correction of the arterial input function reduced the myocardial blood flow by 0.7ml/g/min across all patients.
Introduction
Myocardial perfusion can be assessed using dynamic MRI during first-pass of a contrast agent. Quantitative perfusion is challenging due to (1) respiratory motion, precluding pixel wise quantification, and (2) non-linear relationship between MRI signal and contrast agent concentration.
Respiratory-induced slice misalignment images can be retrospectively corrected using registration.1 However, due to the rapid change in signal intensity during the contrast bolus transit, intensity based registration techniques may not be readily applied. Tracking of the respiratory motion of the heart may be facilitated by separating water and fat signal, using multi-echo Dixon reconstruction. Fat images may then be used to estimate respiratory motion with simple intensity based registration methods which can subsequently be applied to correct the corresponding diagnostic water image.
Secondly, the multi-echo information may be used to better estimate the arterial input function (AIF), by correcting for T2*-related signal loss, and improve perfusion quantification when the main bolus is used as AIF.2 In this study, we propose multi-echo Dixon acquisition for motion correction and AIF estimation for first-pass perfusion.
Methods
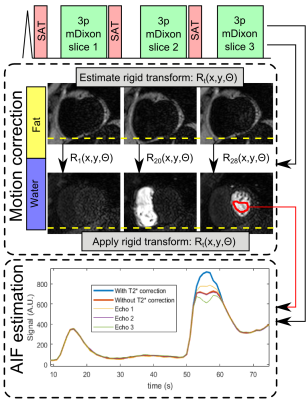
MRI acquisition: The proposed acquisition strategy is shown in Figure 1, and consisted of a spoiled gradient echo readout with three echoes per excitation pulse to enable water-fat separation using Dixon reconstruction.3 Three slices (basal, mid and apical) were acquired per cardiac cycle. Imaging parameters were: Δx=2.3×2.3×10mm, FOV=360×300mm, TR/TE1/ΔTE=3.9/1.05/0.9ms, SENSE=2, partial Fourier=0.63, Tacq=143ms, saturation delay=100ms. The scan was performed at rest over 1 minute during free-breathing with a dual-bolus contrast injection of 0.075mmol/kg.4 8 patients clinically referred for non-stress cardiac MRI assessment were recruited.
Motion correction: Water and fat images were generated using Dixon reconstruction. The fat images were used to estimate respiratory motion. To this end, a rigid-body cross-correlation algorithm was used to calculate displacement and rotation for each frame. Motion correction was performed on the corresponding diagnostic water images by applying the calculated rigid-body transformation for each frame.
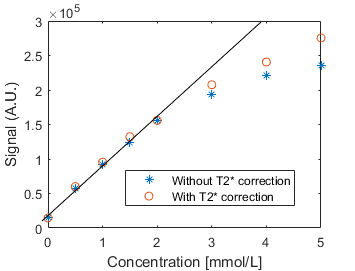
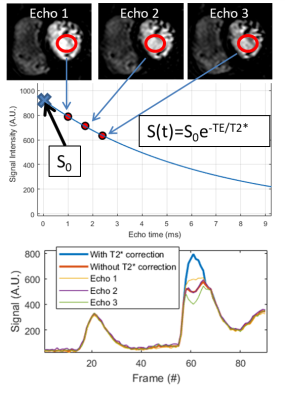
AIF estimation: For each echo, a region-of-interest was defined in the left-ventricle and T2* was estimated by fitting the mean intensity for each echo to S(t)=S0e-TE/T2*, where S0 is the signal without T2* decay. Example signal for the 3 echoes and T2* corrected AIF is shown in Figure 1. A phantom scan with contrast agent concentrations ranging from 0 to 5mmol/L was performed to compare the signal with and without T2* correction.
Perfusion quantification: Perfusion images were manually segmented and pixel-wise time signal intensity curves extracted. Quantitative myocardial blood flow (MBF) was estimated using Fermi model-based deconvolution.5 Quantification was performed using motion corrected and non-motion corrected perfusion images using the main bolus with and without T2* correction. The coefficient of variation of rest perfusion estimates was used to assess the effects of motion compensation on the homogeneity of rest perfusion values.
Results
Signal intensity as a function of contrast agent concentration for the phantom study is shown in Figure 2. Linearity is maintained until 2mmol/L, at higher concentrations signal more closely approximate linearity after T2* correction.
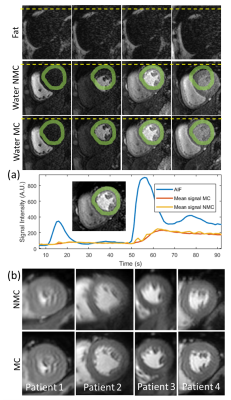
Respiratory motion corrected perfusion scan for one patient is shown in Figure 3a. The proposed approach minimizes the motion-induced signal changes across the scan for the myocardium. Perfusion images from 4 patients, averaged across the whole scan with and without motion correction, are shown in Figure 3b. Improved myocardial sharpness for the image averaged across the scan was obtained in all patients following motion correction.
AIF estimation using
the three echo images for one patient is shown in Figure 4. Mean MBF across all
patients was 2.8±0.5ml/g/min without T2*-corrected AIF, and 2.1±0.3ml/g/min
with T2*-corrected AIF. Motion correction yielded a mean coefficient of variation of 0.22±0.01ml/g/min,
while no motion correction resulted in a coefficient of variation of 0.37±0.08ml/g/min.
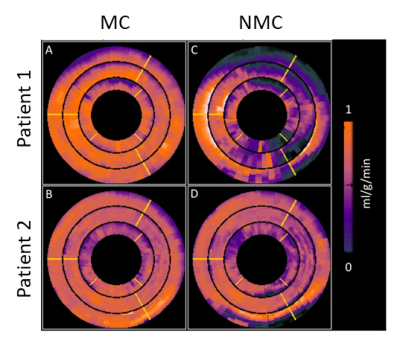
MBF
maps for two patients, with and without respiratory motion correction are shown in
Figure 5.
Discussion
Multi-echo Dixon can be used to address two of the main challenges of quantitative first-pass perfusion: respiratory motion and AIF estimation. Following water-fat separation, the fat images (which are unaffected by the contrast agent bolus) can be used to accurately track respiratory motion with simple and fast image registration techniques. The three echo images enable T2* correction of the AIF to improve quantification. Therefore, with this approach, neither dual-bolus4 nor dual-sequence2 is required for AIF estimation. Optimization of the technique may improve motion correction and AIF estimation. Further validation of this new perfusion approach in patients with ischemia is now warranted.Acknowledgements
This work has been supported by the Brazilian agency CAPES, CEFET-MG, CNPq and FAPEMIG.References
1. Wollny G, Kellman P, Santos A, Ledesma-Carbayo MJ. Automatic motion compensation of free breathing acquired myocardial perfusion data by using independent component analysis. Med Image Anal. 2012 Jul;16(5):1015-28.
2. Kellman P, Hansen MS, Nielles-Vallespin S, et al. Myocardial perfusion cardiovascular magnetic resonance: optimized dual sequence and reconstruction for quantification. J Cardiovasc Magn Reson. 2017;19:43.
3. Eggers H, Brendel B, Duijndam A, Herigault G. Dual-echo Dixon imaging with flexible choice of echo times. Magn Reson Med. 2011 Jan;65(1):96-107.
4. Ishida M, Schuster A, Morton G, et al. Development of a universal dual-bolus injection scheme for the quantitative assessment of myocardial perfusion cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2011 May 24;13:28.
5. Jerosch-Herold M, Wilke N, Stillman AE. Magnetic resonance quantification of the myocardial perfusion reserve with a Fermi function model for constrained deconvolution. Med Phys. 1998;25:73–84.
Figures