0756
The intracellular component of VERDICT (Vascular, Extracellular, and Restricted Diffusion for Cytometry in Tumors) MRI distinguishes Gleason 4 pattern better than Apparent Diffusion Coefficient1Centre of Medical Imaging, UCL, London, United Kingdom, 2Centre for Medical Imaging, UCL, London, United Kingdom, 3UCL, London, United Kingdom, 4UCLH, London, United Kingdom
Synopsis
VERDICT (Vascular, Extracellular, and Restricted Diffusion for Cytometry in Tumours) MRI combines a diffusion-weighted MRI acquisition with a three-compartment mathematical model that describes signal from i) intracellular water (fIC), ii) water in extracellular-extravascular space (fEES) and iii) water in the microvasculature (fvasc). In contrast to VERDICT, clinical ADC is derived from a mono-exponential model. Upon comparison between VERDICT parameters and clinical ADC, we showed that fIC was better able to discriminate between Gleason ≥3+4 histology pattern and Gleason ≤3+3/benign histology. We also showed that image quality of VERDICT-MRI maps and clinical ADC was comparable.
Introduction
Multi-parametric MRI (mpMRI) consists of T2-weighted, diffusion-weighted (DW) imaging with apparent diffusion coefficient (ADC) map and dynamic contrast-enhanced imaging (DCE-MRI)(1). VERDICT-MRI (Vascular, Extracellular, and Restricted Diffusion for Cytometry in Tumours) is a novel quantitative microstructural imaging tool for prostate cancer (2). It combines a five-non-zero-b-value DW-MRI acquisition with a three-compartment mathematical model that describes signal from i) intracellular water (fIC), ii) water in extracellular-extravascular space (fEES) and iii) water in the microvasculature (fvasc) (3). Clinical ADC is derived from a mono-compartmental model and single non-zero b-value. Clinical ADC conflates parameters like cell density, size, permeability, subcellular structure and vascular perfusion, which may cancel each other’s effects, while VERDICT aims to quantify them separately thus enhancing sensitivity and specificity (2). We previously assessed the repeatability of VERDICT parameters and their correlation to histology (4). The aims of this study are: (a) to compare the accuracy of each VERDICT parameter (fIC, fEES, fvasc), ADC and early-enhancement T1-weighted DCE-MRI to distinguish between presence of Gleason ≥3+4 and Gleason ≤6/benign histology and (b), compare image quality of VERDICT parameters and ADC.Methods
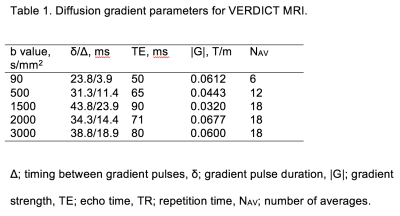
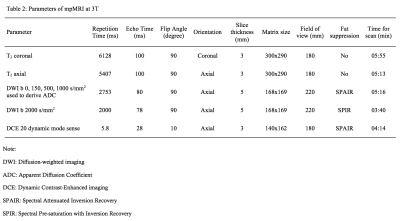
62 men with suspected prostate cancer or undergoing active surveillance were recruited as part of a prospective trial. They all had a 3T prostate mpMRI and VERDICT-MRI (protocols shown in Tables 1&2). Thirty-eight men with a focal prostate lesion identified as PI-RADS 3, 4 or 5 (1) underwent targeted transperineal template biopsy. Figure 1 illustrates a prostate mpMRI and VERDICT-MRI study.
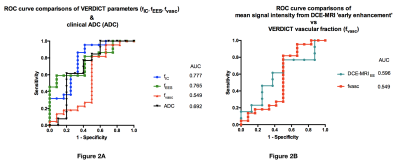
A board-certified radiologist manually contoured a region-of-interest (ROI) for each focal index lesion (i.e the most suspicious lesion) on the ADC map, DCE-MRI and VERDICT-MRI parametric maps (fIC, fEES, fvasc). Quantitative mean values of ADC, fIC, fEES, fvasc and the mean signal intensity of early-enhanced DCE-MRI (DCE-MRIEE) were obtained. The reference standard was histology from targeted transperineal template biopsy. The cohort (n=38) was divided into two groups: Gleason ≥3+4 pattern and Gleason ≤3+3/benign (5). Receiver-operator characteristic curves (ROC) were plotted and area-under-the-curve (AUC) calculated for each parameter.
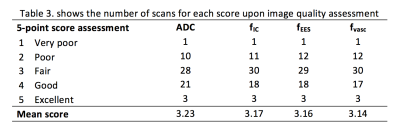
To compare image quality of VERDICT maps (fIC, fEES, fvasc) and ADC, two board-certified radiologists and an MRI physicist qualitatively assessed in consensus each map for the 62 patients. Influence of image artifact on diagnostic quality was scored using a subjective 1-5 ordinal scale (6) where score 1: very poor quality, non-diagnostic (artifacts on all slices, scans uninterpretable); 2: poor quality with some impairment of diagnostic quality (substantial artifacts, but still interpretable); 3: satisfactory quality without impairment of diagnostic quality (some artifacts present); 4: good quality (hardly any artifacts); 5: excellent quality (no artifacts present). The Freidman test with Dunn’s multiple comparison correction was performed to determine the differences between overall image quality.
Results
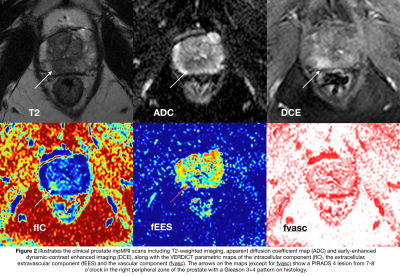
Of the 38 patients with biopsy results, 23 harbored Gleason ≥3+4 pattern. The ROC curves comparing each VERDICT diffusion parameter to discriminate between histology Gleason ≥3+4 and Gleason ≤3+3/benign are shown in Figure 2. The AUC of fIC, fEES, fvasc were 0.777, 0.765 and 0.549 respectively. Those of ADC and DCE-MRIEE were 0.692 and 0.596 respectively. Table 2 summarizes the results for the image quality assessment of VERDICT parameters and ADC.Discussion
Our study showed that the intracellular fraction of VERDICT MRI (fIC) had the highest AUC compared to the other VERDICT and clinical mpMRI parameters in distinguishing between Gleason score ≥3+4 and Gleason score ≤3+3/benign histology. We also showed that the mean score of image quality is very similar amongst all VERDICT parameters and clinical ADC with no statistical difference found.
The ability of VERDICT fIC to make the distinction between the presence/absence of Gleason 4 component is of clinical relevance as it may represent a potential non-invasive biomarker in prostate cancer management including active surveillance to better select patients who could avoid a biopsy. Its application to clinical use would necessitate validation in clinical studies.
The added complexity of the VERDICT model did not contribute to lowering the image quality of the VERDICT maps. Image quality in VERDICT parametric maps was similar to ADC. This is because the voxel size was the same and so the spatial resolution of these maps was equivalent. Also, same echo-planar based sequences were used, lending to the same causes of image artifact. The mean scores of ADC image quality in our cohort (3.16-3.23) were comparable to those published in a previous study (3.03-3.08)(7).
Conclusion
Our study demonstrates that the intracellular fraction of VERDICT MRI (fIC) was most able to distinguish between Gleason score ≥3+4 and Gleason score ≤3+3/benign histology. We also found that there is no difference in image quality between each VERDICT parameter and clinical ADC.Acknowledgements
This work was undertaken at UCLH/ UCL, which received a proportion of the funding from the NIHR Biomedical Research Centres funding scheme of the UK Department of Health. This work is funded by a grant PG14-018-TR2 from Prostate Cancer UK.References
1. Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol. 2016;69(1):16-40.
2. Panagiotaki E, Chan RW, Dikaios N, Ahmed HU, O'Callaghan J, Freeman A, et al. Microstructural characterization of normal and malignant human prostate tissue with vascular, extracellular, and restricted diffusion for cytometry in tumours magnetic resonance imaging. Invest Radiol. 2015;50(4):218-27.
3. Panagiotaki E, Walker-Samuel S, Siow B, Johnson SP, Rajkumar V, Pedley RB, et al. Noninvasive quantification of solid tumor microstructure using VERDICT MRI. Cancer Res. 2014;74(7):1902-12.
4. Johnston WE, Bonet-Carne E, Pye H, Clemente J, Yvernault B, Patel D, et al., editors. Microstructural Diffusion-Weighted (VERDICT) MRI Metrics are Repeatable and Show Potential at Characterising Gleason 7 Prostate Cancer Non-Invasively. The International Society for Magnetic Resonance in Medicine; 2017; Honolulu, Hawaii.
5. Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA, et al. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Pathol. 2016;40(2):244-52.
6. Rensis L. A technique for the measurement of attitudes. Archives of Psychology. 1932;22(1932-1933):5-55.
7. Barth BK, Cornelius A, Nanz D, Eberli D, Donati OF. Comparison of image quality and patient discomfort in prostate MRI: pelvic phased array coil vs. endorectal coil. Abdom Radiol (NY). 2016;41(11):2218-26.