0754
Anatomy-constrained automated fiber tract reconstruction for surgery planning: a validation study in language-related white matter tracts1Translational Imaging Group, University College London, London, United Kingdom, 2Wellcome EPSRC Centre for Interventional and Surgical Sciences (WEISS), University College London, London, United Kingdom, 3Epilepsy Society MRI Unit, Chalfont St Peter, United Kingdom, 4Department of Clinical and Experimental Epilepsy, University College London, London, United Kingdom, 5Neuroimaging of Epilepsy Laboratory, Montreal Neurological Institute, McGill University, Montreal, Canada, 6Dementia Research Centre, University College London, London, United Kingdom
Synopsis
Diffusion MRI and tractography hold great potential for surgery planning, but fiber tract reconstruction requires an expert rater. In this work, we set up an automated reconstruction pipeline based on anatomical criteria that does not require manual intervention and we validated it on epilepsy patients with specific focus on language-related bundles. We first compared the results with the ones obtained from human raters and then further validated them using task fMRI. The fiber tracts reconstructed from the pipeline were in line with the agreement between different human raters and showed good overlap with function.
Introduction
The use of diffusion-weighted imaging (DWI) and tractography in surgery planning is still limited, despite their potential to inform the surgeons on function-related white matter bundles to avoid during resections1. Although several semi-automated approaches have been proposed in the last years2,3, they all require expert manual segmentation of the tracts. Additionally, validation of such methods has been mostly on healthy subjects with scarce attention to function-related aspects. Here we propose an automated parcellation-based approach that relies on anatomically constrained tractography (ACT) and validate its application on reconstructing language-related tracts of temporal lobe epilepsy (TLE) patients.Methods
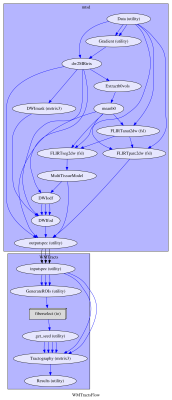
The dataset consists of thirty TLE patients (mean age(SD): 36.87(11.41); m/f: 12/18; affected lobe: 14 left/16 right). These patients were scheduled for resection and underwent the MRI protocol as part of the clinical procedures, that included: T1-weighted sequence (MPRAGE) and multi-shell DWI (2mm isotropic resolution, gradient directions: 11, 8, 32, and 64 at b-values: 0, 300, 700, and 2500 s/mm2, single b=0-image with reverse phase-encoding). The patients also underwent task-based fMRI (gradient-echo planar T2*-weighted images with TE/TR = 22/2500 ms, 50 contiguous 2.4mm slices (0.1mm gap) with a 24 cm field of view, 64×64 matrix, in-plane pixel size of 3.75×3.75 mm). Three tasks were employed: auditory naming (AN), picture naming (PN) and verbal fluency (VF)4,5. The acquired data were processed using a tailored pipeline assembled with NiPype (fig.1). Briefly, T1-weighted data were processed using geodesic-information flow (GIF) for tissue segmentation and parcellation6. Then, segmentation and parcellation data were rigidly co-registered in the diffusion space. DWI data were corrected for signal drift, geometric distortions and eddy-current induced distortions. An orientation distribution function was estimated using multi-tissue constrained spherical deconvolution7. Fiber tracts were reconstructed probabilistically with MRTrix3 using iFOD2 and ACT: focusing on the bundles related to language, we reconstructed the arcuate fasciculus (AF), inferior fronto-occipital fasciculus (IFOF), inferior longitudinal fasciculus (ILF), and uncinate fasciculus (UF) bilaterally. Using GIF parcellation, the seeding regions as well as the inclusion and exclusion areas were defined based on anatomical criteria8. 5000 streamlines were estimated randomly placing the seeds at the white matter/grey matter interface. To exclude spurious streamlines, a cluster-based approach based on QuickBundles was used9, discarding streamlines with either direction, distance or position of the centre of gravity out of the main clusters. We adopted a two-fold validation approach: as a first validation, for ten of the thirty subjects (5 RTLE/5 LTLE) the fiber tracts of the left hemisphere were manually segmented by two experts, following established criteria10. Using the Cohen’s kappa and the Jaccard index10,2, we quantified agreement and overlap between the automated procedure and the manual ones and compared it to the inter-rater agreement. As a further validation, we used the maximal activation points in the language-dominant hemisphere obtained from the fMRI tasks as seeds for probabilistic tractography for all the subjects. Using the mentioned scores, we quantified the overlap between the language-related fiber tracts and the ones obtained using fMRI seeding.Results
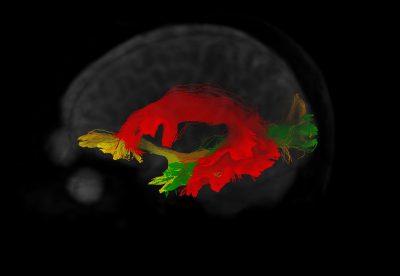
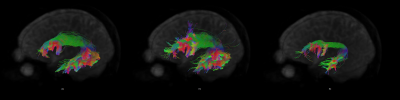
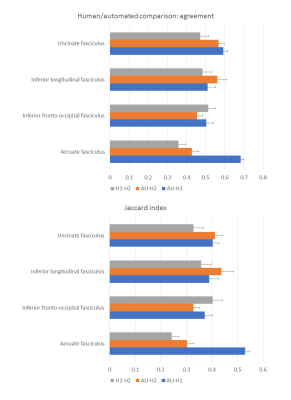
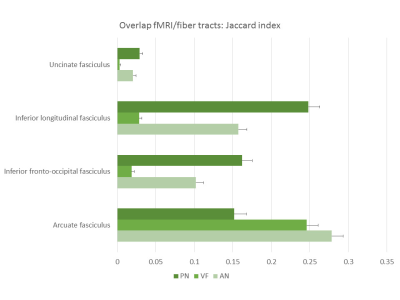
Figure 2 shows the tracts for a sample subject on the left hemisphere, while figure 3 shows the left AF for the same subject as reconstructed from the automated procedure and the human raters. The kappa showed in general moderate agreement between the automated reconstructed tracts and the expert ones, with AF getting substantial agreement with one rater (fig.4). It must be noticed that in all the comparisons the agreement was greater or comparable to the inter-rater agreement. The Jaccard index showed similar results. The overlap between fMRI seeded and automated reconstructed tracts highlighted mainly good overlap between AF and all the tasks and scarce overlap with UF (fig.5).Discussion
The automated procedure reconstructed good quality tracts with few spurious streamlines. Compared to the human experts, the agreement differed between the raters, but showed higher values than the inter-rater comparison, indicating it finds a good middle ground between different human raters. When compared to previous work2, the overlap in terms of Jaccard index showed comparable or higher values than the ones reported in Garyfallidis and colleagues2. In terms of functional overlap, the VF task activated the inferior and middle frontal gyrus5, and we consistently observed a predominant overlap with AF. PN and AN tasks respectively activated superior and middle temporal gyri4, and as a consequence the related tracts overlap in different ways with IFOF, ILF and AF. The UF was not involved in the tasks and accordingly it scarcely overlaps.Conclusions
We proposed an anatomy-constrained method for reconstructing fiber tracts that does not require an expert eye. We observed results comparable to human ones and functionally meaningful.Acknowledgements
We are grateful to the Wolfson Foundation and Epilepsy Society for supporting the Epilepsy Society MRI
scanner. This work was supported by the Wellcome/EPSRC (203145Z/16/Z), NIHR BRC UCLH/UCL High Impact
Initiative, MRC (MR/M00841X/1), and Health Innovation Challenge Fund (WT106882).
KT was supported by a 1-year fellowship each by the European Academy of Neurology and the Austrian Society of Neurology.
References
1. Essayed WI, Zhang F, Unadkat P, et al. White matter tractography for neurosurgical planning: A topography-based review of the current state of the art. NeuroImage Clinical. 2017;15:659-72.
2. Garyfallidis E, Cote MA, Rheault F, et al. Recognition of white matter bundles using local and global streamline-based registration and clustering. NeuroImage. 2017.
3. Wassermann D, Makris N, Rathi Y, et al. The white matter query language: a novel approach for describing human white matter anatomy. Brain structure & function. 2016;221(9):4705-21.
4. Gonzalvez GG, Trimmel K, Haag A, et al. Activations in temporal areas using visual and auditory naming stimuli: A language fMRI study in temporal lobe epilepsy. Epilepsy research. 2016;128:102-12.
5. Bonelli SB, Powell R, Thompson PJ, et al. Hippocampal activation correlates with visual confrontation naming: fMRI findings in controls and patients with temporal lobe epilepsy. Epilepsy research. 2011;95(3):246-54.
6. Cardoso MJ, Modat M, Wolz R, et al. Geodesic Information Flows: Spatially-Variant Graphs and Their Application to Segmentation and Fusion. IEEE transactions on medical imaging. 2015;34(9):1976-88.
7. Jeurissen B, Tournier JD, Dhollander T, et al. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. NeuroImage. 2014;103:411-26.
8. Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex; a journal devoted to the study of the nervous system and behavior. 2008;44(8):1105-32.
9. Garyfallidis E, Brett M, Correia MM, et al. QuickBundles, a Method for Tractography Simplification. Frontiers in neuroscience. 2012;6:175.
10. Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage. 2007;36(3):630-44.
Figures