0752
Evaluation of Standardized and Study-Specific Diffusion Tensor Imaging Templates of the Adult Human Brain1Biomedical Engineering, Illinois Institute of Technology, Chicago, IL, United States
Synopsis
DTI templates of the adult human brain are commonly used in neuroimaging, and their characteristics influence the accuracy of the application. The purpose of this work was to compare eight available standardized DTI templates to each other, as well as to study-specific templates, in terms of template characteristics and performance in spatial normalization and detection of small inter-group FA differences. The IIT v.3.0 template was shown to combine a number of desirable characteristics, and allows higher inter-subject spatial normalization accuracy and detection of smaller inter-group FA differences, compared to other templates, including study-specific templates, for both younger and older adults.
Introduction
Diffusion tensor imaging (DTI) templates of the adult human brain are commonly used in neuroimaging research, and their characteristics influence the accuracy of the application. However, a systematic evaluation of the characteristics and performance of standardized and study-specific DTI templates has not been conducted. The purpose of this work was to compare eight available standardized DTI templates to each other, as well as to study-specific templates, in terms of template characteristics and performance in spatial normalization and detection of small inter-group FA differences.Methods
Two 3T datasets from the Enhanced Nathan Kline Institute Rockland Sample (http://fcon_1000.projects.nitrc.org/indi/enhanced/sharing.html) were used in this work. Dataset 1 included DTI and T1-weighted data from 72 healthy young and middle-aged adults (18-59 years of age), and Dataset 2 included DTI and T1-weighted data from 72 healthy older adults (65-85 years of age), collected using SE-EPI-DTI (2x2x2 mm3, b=1,500 s/mm2, 128 diffusion directions, nine b=0 s/mm2 volumes) and 3D MPRAGE (1x1x1 mm3). DTI processing was conducted using TORTOISE1.
Eight standardized DTI templates of the adult human brain and two study-specific templates were compared. The standardized templates included five providing full tensor information: IIT v.3.02, IIT23, ICBM814, Eve5, and NTU-DSI-122-DTI6; and three providing only tensor-derived scalar quantities: ENIGMA7, FMRIB58 (FMRIB), and SRI248. The two study-specific diffusion tensor templates were constructed based on Datasets 1 (SS-y) and 2 (SS-o), separately, using a state-of-the-art approach (DTI-TK9).
A) FA maps of all templates were compared in terms of image sharpness, ability to identify small brain structures, and artifacts content.
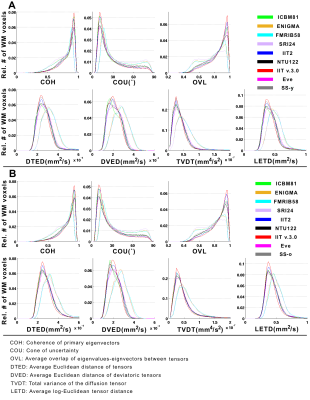
B) The accuracy of inter-subject DTI spatial normalization achieved when using each of the templates as reference was compared across templates, for normalization of younger (Dataset 1) and older (Dataset 2) adults, separately. For templates providing full tensor information, tensor-based registration was conducted using DTI-TK9-14. For templates providing only tensor-derived scalar quantities, FA-based registration was conducted using ART15,16, followed by finite strain tensor reorientation17. A number of metrics were used to assess the accuracy of inter-subject spatial normalization voxel-wise. Histograms of these quantities in white matter were generated for Datasets 1 and 2, separately, and were compared across templates using the one-sided two-sample Kolmogorov-Smirnov (KS) test.
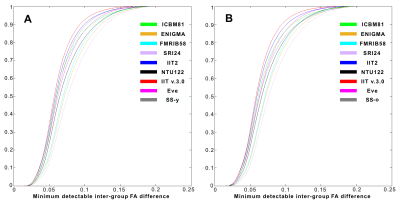
C) Power analysis was used to assess the impact of spatial normalization accuracy on the ability to detect small inter-group FA differences (assuming two groups, 100 participants per group, significance at p<0.05, power>0.95). Maps of the minimum detectable inter-group FA differences were generated for all templates, and corresponding cumulative distributions were compared across templates using the one-sided two-sample KS test.
Results
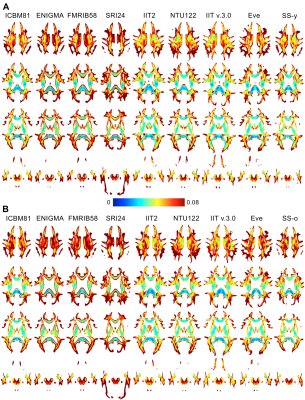
A) Image sharpness was higher in the FA maps of Eve and IIT v.3.0 compared to other templates (Fig.1). Fine white matter details were observed throughout the brain in IIT v.3.0 and Eve templates, while the same information was often lost in other templates (Fig.2A). Image artifacts were present in ICBM81, Eve, NTU-DSI-122-DTI, and SRI24 (Fig.2B).
B) The accuracy of inter-subject spatial normalization was higher when using the IIT v.3.0 template compared to all other templates, including the corresponding study-specific templates, for both younger (Fig.3A) and older adults (Fig.3B)(p<10-8 in all cases).
C) Power analysis showed that, on average over all white matter, the IIT v.3.0 template allowed detection of smaller inter-group FA differences compared to other templates, including the study-specific templates, for both younger (Figs.4A,5A) and older adults (Figs.4B,5B)(p<10-8 in all cases).
Discussion
The IIT v.3.0 template combines a number of desirable characteristics over other templates: full-tensor information, population-based, high image sharpness, no visible artifacts, features representative of individual adult brains. It was demonstrated that the IIT v.3.0 template allows higher inter-subject DTI spatial normalization accuracy, and detection of smaller inter-group FA differences, compared to other templates, including study-specific templates, for both younger and older adults.
At first glance, the results showing higher spatial normalization accuracy when using a standardized instead of a study-specific template appear to be in conflict with the work by van Hecke et al.18, which established the use of study-specific templates as the modus operandi in DTI investigations. However, van Hecke et al. only compared a study-specific template and a single standardized template, namely ICBM81. The present work is actually in agreement with the findings by van Hecke et al. Moreover, the present work substantially extends that of van Hecke et al.18 by considering standardized templates that were not available in 2011, and shows that IIT v.3.0, a standardized template, can lead to higher spatial normalization accuracy than study-specific templates constructed with state-of-the-art approaches. This result is of high significance as it pertains to the template selection strategy used in many DTI studies.
Acknowledgements
This work was supported by grants from the National Institute of Biomedical Imaging and Bioengineering (NIBIB) (R21EB006525), the National Institute of Neurological Disorders and Stroke (NINDS) (R21NS076827), and the National Institute of Aging (NIA) (R01AG052200).References
[1] C. Pierpaoli, L. Walker, M. O. Irfanoglu, A. Barnett, P. Basser, L.-C. Chang, C. Koay, S. Pajevic, G. Rohde, J. Sarlls, and M. Wu, “TORTOISE: an integrated software package for processing of diffusion MRI data,” in ISMRM Annual Meeting Proceedings, 2010, p. 1597.
[2] A. Varentsova, S. Zhang, and K. Arfanakis, “Development of a high angular resolution diffusion imaging human brain template,” Neuroimage, vol. 91, pp. 177–186, 2014.
[3] S. Zhang, H. Peng, R. J. Dawe, and K. Arfanakis, “Enhanced ICBM diffusion tensor template of the human brain.,” Neuroimage, vol. 54, no. 2, pp. 974–84, 2011.
[4] S. Mori, K. Oishi, H. Jiang, L. Jiang, X. Li, K. Akhter, K. Hua, A. V Faria, A. Mahmood, R. Woods, A. W. Toga, G. B. Pike, P. R. Neto, A. Evans, J. Zhang, H. Huang, M. I. Miller, P. van Zijl, and J. Mazziotta, “Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template.,” Neuroimage, vol. 40, no. 2, pp. 570–82, Apr. 2008.
[5] K. Oishi, A. Faria, H. Jiang, X. Li, K. Akhter, J. Zhang, J. T. Hsu, M. I. Miller, P. C. M. van Zijl, M. Albert, C. G. Lyketsos, R. Woods, A. W. Toga, G. B. Pike, P. Rosa-Neto, A. Evans, J. Mazziotta, and S. Mori, “Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer’s disease participants.,” Neuroimage, vol. 46, no. 2, pp. 486–99, Jun. 2009.
[6] Y.-C. Hsu, Y.-C. Lo, Y.-J. Chen, V. J. Wedeen, and W.-Y. Isaac Tseng, “NTU-DSI-122: A diffusion spectrum imaging template with high anatomical matching to the ICBM-152 space.,” Hum. Brain Mapp., vol. 36, no. 9, pp. 3528–41, Sep. 2015.
[7] N. Jahanshad, P. V Kochunov, E. Sprooten, R. C. Mandl, T. E. Nichols, L. Almasy, J. Blangero, R. M. Brouwer, J. E. Curran, G. I. de Zubicaray, R. Duggirala, P. T. Fox, L. E. Hong, B. A. Landman, N. G. Martin, K. L. McMahon, S. E. Medland, B. D. Mitchell, R. L. Olvera, C. P. Peterson, J. M. Starr, J. E. Sussmann, A. W. Toga, J. M. Wardlaw, M. J. Wright, H. E. Hulshoff Pol, M. E. Bastin, A. M. McIntosh, I. J. Deary, P. M. Thompson, and D. C. Glahn, “Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: a pilot project of the ENIGMA-DTI working group.,” Neuroimage, vol. 81, pp. 455–69, Nov. 2013.
[8] T. Rohlfing, N. M. Zahr, E. V Sullivan, and A. Pfefferbaum, “The SRI24 multichannel atlas of normal adult human brain structure.,” Hum. Brain Mapp., vol. 31, no. 5, pp. 798–819, May 2010.
[9] H. Zhang, P. A. Yushkevich, D. C. Alexander, and J. C. Gee, “Deformable registration of diffusion tensor MR images with explicit orientation optimization.,” Med. Image Anal., vol. 10, no. 5, pp. 764–85, Oct. 2006.
[10] Y. Wang, Q. Yu, Z. Liu, T. Lei, Z. Guo, M. Qi, and Y. Fan, “Evaluation on diffusion tensor image registration algorithms,” Multimed. Tools Appl., Jun. 2015.
[11] Y. Wang, A. Gupta, Z. Liu, H. Zhang, M. L. Escolar, J. H. Gilmore, S. Gouttard, P. Fillard, E. Maltbie, G. Gerig, and M. Styner, “DTI registration in atlas based fiber analysis of infantile Krabbe disease.,” Neuroimage, vol. 55, no. 4, pp. 1577–86, Apr. 2011.
[12] S. Zhang and K. Arfanakis, “Role of standardized and study-specific human brain diffusion tensor templates in inter-subject spatial normalization,” J. Magn. Reson. Imaging, vol. 37, no. 2, pp. 372–381, 2013.
[13] P. Kochunov, N. Jahanshad, D. Marcus, A. Winkler, E. Sprooten, T. E. Nichols, S. N. Wright, L. E. Hong, B. Patel, T. Behrens, S. Jbabdi, J. Andersson, C. Lenglet, E. Yacoub, S. Moeller, E. Auerbach, K. Ugurbil, S. N. Sotiropoulos, R. M. Brouwer, B. Landman, H. Lemaitre, A. den Braber, M. P. Zwiers, S. Ritchie, K. van Hulzen, L. Almasy, J. Curran, G. I. deZubicaray, R. Duggirala, P. Fox, N. G. Martin, K. L. McMahon, B. Mitchell, R. L. Olvera, C. Peterson, J. Starr, J. Sussmann, J. Wardlaw, M. Wright, D. I. Boomsma, R. Kahn, E. J. C. de Geus, D. E. Williamson, A. Hariri, D. van ’t Ent, M. E. Bastin, A. McIntosh, I. J. Deary, H. E. Hulshoff Pol, J. Blangero, P. M. Thompson, D. C. Glahn, and D. C. Van Essen, “Heritability of fractional anisotropy in human white matter: a comparison of Human Connectome Project and ENIGMA-DTI data.,” Neuroimage, vol. 111, pp. 300–11, May 2015.
[14] M. O. Irfanoglu, A. Nayak, J. Jenkins, E. B. Hutchinson, N. Sadeghi, C. P. Thomas, and C. Pierpaoli, “DR-TAMAS: Diffeomorphic Registration for Tensor Accurate Alignment of Anatomical Structures.,” Neuroimage, vol. 132, pp. 439–54, May 2016.
[15] B. A. Ardekani, S. Guckemus, A. Bachman, M. J. Hoptman, M. Wojtaszek, and J. Nierenberg, “Quantitative comparison of algorithms for inter-subject registration of 3D volumetric brain MRI scans.,” J. Neurosci. Methods, vol. 142, no. 1, pp. 67–76, Mar. 2005.
[16] A. Klein, J. Andersson, B. A. Ardekani, J. Ashburner, B. Avants, M.-C. Chiang, G. E. Christensen, D. L. Collins, J. Gee, P. Hellier, J. H. Song, M. Jenkinson, C. Lepage, D. Rueckert, P. Thompson, T. Vercauteren, R. P. Woods, J. J. Mann, and R. V Parsey, “Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration.,” Neuroimage, vol. 46, no. 3, pp. 786–802, Jul. 2009.
[17] D. C. Alexander, C. Pierpaoli, P. J. Basser, and J. C. Gee, “Spatial transformations of diffusion tensor magnetic resonance images.,” IEEE Trans. Med. Imaging, vol. 20, no. 11, pp. 1131–9, Nov. 2001.
[18] W. Van Hecke, A. Leemans, C. A. Sage, L. Emsell, J. Veraart, J. Sijbers, S. Sunaert, and P. M. Parizel, “The effect of template selection on diffusion tensor voxel-based analysis results.,” Neuroimage, vol. 55, no. 2, pp. 566–73, Mar. 2011.
Figures
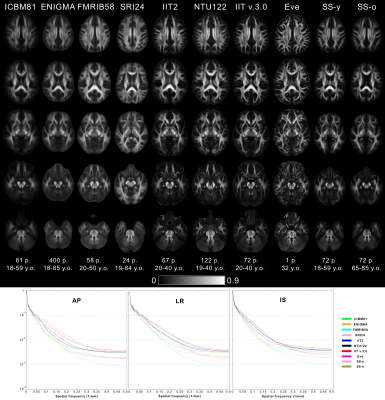
Figure 1. Columns of FA maps for five axial brain slices of the ICBM81, ENIGMA, FMRIB58, SRI24, IIT2, NTU-DSI-122-DTI, IIT v.3.0, Eve, SS-y and SS-o templates. The number and age-range of persons considered in the construction of each template are shown below each column.
(Bottom row) Normalized power spectra of FA maps for the anterior-posterior (AP), left-right (LR), and inferior-superior (IS) axes, separately, from all templates. The energy at high spatial frequencies was higher in the normalized power spectra of FA maps from Eve and IIT v.3.0 compared to other DTI templates, on average over all axes.
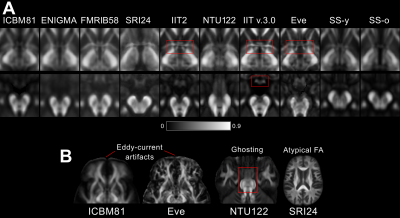
Figure 2. A) Fine white matter details in the area of the anterior commissure (top row) are visible in IIT v.3.0, Eve and IIT2 templates, while, in contrast, the anterior commissure is indistinguishable from the column and pre-commissural part of the fornix in ENIGMA, SRI24, SS-y, and SS-o, and not present in ICBM81 and NTU-DSI-122-DTI. The optic chiasm (bottom row) is well-defined in IIT v.3.0, while it is not discernible in NTU-DSI-122-DTI, ENIGMA, or SS-o.
B) Artifacts. Eddy-current-induced artifacts in the frontal lobe of ICBM81 and Eve. Ghosting of the pons in NTU-DSI-122-DTI. Atypical FA map in SRI24.