0747
Selective degeneration of crossing fibres and its relationship with fractional anisotropy1Rotman Research Institute, Baycrest Health Sciences, Toronto, ON, Canada, 2Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 3Departments of Psychiatry and Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, United States, 4MGH/HST Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA, United States
Synopsis
Selective degeneration of crossing fibres has been reported in diffusion MRI studies of Alzheimer’s disease alongside increased fractional anisotropy (FA). Similar albeit more subtle selective degeneration has been suggested in healthy aging, but increased FA with age has not been reported on cross-sectional studies of aging. In this work, we use DTI tractography to measure selective degeneration of crossing fibres in healthy aging among 212 subjects. Increased FA with age is found in this cohort only after applying free water elimination (FWE), suggesting that increasing extracellular water in aging may introduce variability that obscure finer structural phenomena in cross-sectional designs.
Introduction
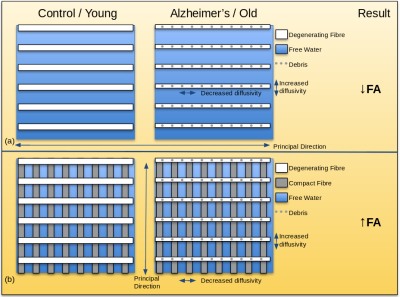
The corona radiata and internal capsule are complex structures of crossing fibres.1 Sensorimotor fibres such as the corticospinal tract project along the principal direction, and are crossed by the superior longitudinal fasciculus in the corona radiata and the thalamic radiations in the internal capsule. In Alzheimer’s disease, sensorimotor fibres have been shown to remain relatively spared while the crossing fibres degenerate.2 Increased FA has been reported in these regions in DTI studies of Alzheimer’s subjects,2-4 interpreted as degeneration of the secondary crossing fibres while the principal fibres remain intact (Figure 1). Similar albeit more subtle selective degeneration of crossing fibres has been suggested in healthy aging.5 Increased FA is generally not reported in healthy aging, although it was recently seen in a longitudinal study involving >500 participants.6 Thus far, such findings have not been reported in cross-sectional DTI studies of aging as the effect may be obscured by inter-subject variability. However, in our previous work, increased FA with healthy aging was found in these regions after applying free water elimination (FWE).7 In the following, we attempt to validate this observation by examining the effect of healthy aging on the proportion of primary and secondary fibres in these regions.Methods
212 healthy subjects aged 39.1 to 91.7 years (62.0 + 11.2) were imaged on a 3T Siemens Magnetom Trio system with a 12-channel head coil. Diffusion MRI (dMRI) with 60 directions was performed at b=700mm2/s following 10 b=0 volumes. Other parameters were TE=83ms, TR=7.98s, FOV=25.6cm, 2mm3 resolution, 70 slices. All dMRI data sets were corrected for motion and eddy currents using eddy_correct8 in FSL,9 and masked using FSL's Brain Extraction Toolbox (bet).10 Diffusion Tensor Imaging (DTI) was performed both conventionally and with free water elimination.11 Voxelwise statistical analysis was conducted in the white matter by extracting a WM skeleton using FSL's Tract-Based Spatial Statistics (TBSS)12. A relationship of DTI parameters with age was defined by p<0.05 with correction for multiple comparisons and controlling for gender as per FSL’s Threshold Free Cluster Enhancement (TFCE). Effect size was calculated via FreeSurfer13 using a general linear model. Regions of interest were assessed using an MNI White Matter Atlas (John Hopkins University). FSL’s bedpostx tool14 was used to compute the estimated proportion of primary (mean_F1samples) and secondary (mean_F2samples) fibre within each voxel. These two parameters underwent the same statistical analysis versus age as above. All images are displayed in RAS (neurological) orientation.Results
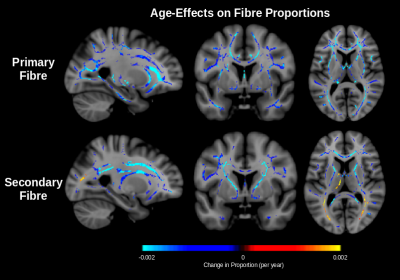
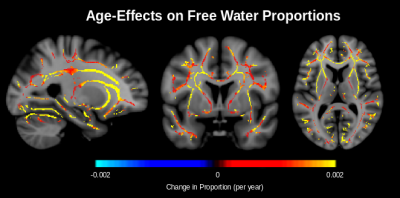
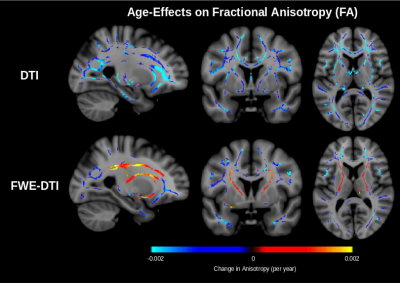
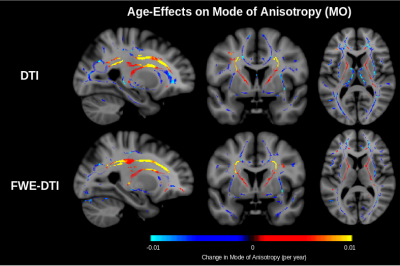
Figure 2 shows that the proportion of both primary and secondary fibres decrease throughout the white matter. In the corona radiata and internal capsule, only the proportion of secondary fibre decreases with age while the proportion of primary fibre remains stable. As reported in our previous work,7 the proportion of free water increases with age (Figure 3). In conventional DTI, FA is found to decrease throughout the white matter with age with minimal change in the corona radiata and internal capsule. After FWE, FA is found to increase in these regions (Figure 4). Additionally, mode of anisotropy (MO) is found to increase with age in both conventional DTI and after FWE (Figure 5).Discussion
This study appears to be the first of healthy aging to show stable proportion of primary fibre with age and decreased proportion of secondary fibre with age in crossing-fibre regions. Such selective degeneration is reflected by increased MO with age, as has recently been reported.5 While this should lead to increased FA with age (Figure 1), isotropic free water (presumably interstitial) also increases with age (Figure 3), apparently masking this effect in conventional DTI. Increased FA with age is found after applying FWE. The only report of this increased FA in a conventional DTI study of normal aging comes from a recent large-scale longitudinal study (N = 501).6 The fact that this has not been reported in cross-sectional studies of healthy aging suggests that the effect may usually be masked by inter-subject variability associated with free water, and that FWE is necessary for elevating this effect to the level of significance achievable in longitudinal studies.Conclusions
While selective degeneration of crossing fibres has been shown in Alzheimer’s disease, this study shows that similar albeit more subtle selective degeneration occurs in the same regions in healthy aging. Moreover, FWE was needed to observe the corresponding increased FA with age. Our work suggests that FWE reduces variability in cross-subject FA to allow for observation of finer structural phenomena, and continuing to re-examine DTI data by accounting for free water can potentially elucidate information on crossing fibres which cannot be observed in conventional DTI.Acknowledgements
We thank the Canadian Institutes of Health Research (CIHR) and the National Institutes of Health (NIH) for financial support.References
1. Catani M, Thiebaut De Schotten M (2012) Atlas of Human Brain Connections. Oxford: Oxford University Press
2. Doan NT, et al (2017) Dissociable diffusion MRI patterns of white matter microstructure and connectivity in Alzheimer's disease spectrum. Sci Rep 7:45131
3. Douaud G, et al (2011) DTI measures in crossing-fibre areas: Increased diffusion anisotropyreveals early white matter alteration in MCI and mild Alzheimer’s disease. Neuroimage 55(3):880-890
4. Teipel SJ, et al (2014) Fractional anisotropy changes in Alzheimer's disease depend on the underlying fiber tract architecture: A multiparametric DTI study using joint independent component analysis. J Alzheimers Dis 41(1):69-83
5. Cox SR, et al (2016) Ageing and brain white matter structure in 3,513 UK Biobank participants. Nat Commun 7:13629
6. de Groot M, et al (2016) White matter degeneration with aging: Longitudinal diffusion MR imaging analysis. Radiology 279(2):532-541
7. Chad J, Pasternak O, Salat DH, Chen JJ (2017) White matter microstructural changes in healthy aging: The effect of free water elimination on DTI metrics. ISMRM 2017.
8. Jenkinson M, Smith S (2001) A global optimisation method for robust affine registration ofbrain images. Med Image Anal 5(2):143-156
9. Smith SM, et al (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 Suppl 1:S208-219
10. Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17(3):143-155
11. Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y (2009) Free water elimination and mapping from diffusion MRI. Magn Reson Med 62:717-730
12. Smith SM, et al (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31(4):1487-1505
13. Fischl B (2012) FreeSurfer. Neuroimage 62(2):774-781
14. Jbabdi S, Behrens TE, Smith SM (2010) Crossing fibres in tract-based spatial statistics. Neuroimage 49:249-256
Figures