0744
Validation of dentato-rubro-thalamic tract in squirrel monkey brain1Biomedical Engineering, Vanderbilt University, Nashville, TN, United States, 2Institute of Imaging Science, Vanderbilt University, Nashville, TN, United States, 3Psychology, Vanderbilt University, Nashville, TN, United States, 4Physics and Astronomy, Vanderbilt University, Nashville, TN, United States, 5Radiation Oncology, Vanderbilt University Medical Center, Nashville, TN, United States, 6Electrical Engineering and Computer Science, Vanderbilt University, Nashville, TN, United States, 7Neurological Surgery, Vanderbilt University Medical Center, Nashville, TN, United States, 8Neurology, Vanderbilt University Medical Center, Nashville, TN, United States
Synopsis
The dentato-rubro-thalamic-tract (DRTT) has recently been suggested as a target for tremor control in deep brain stimulation and stereotactic radiosurgery, however, its efficacy has been challenged because different approaches to diffusion MRI (dMRI) tractography exhibited significantly different sensitivity in detecting the DRTT. We implemented a framework to quantitatively evaluate the performance of dMRI tractography by comparing the dMRI tracts to the histological DRTT identified from Nissl-stained sections in the same squirrel monkey brain. The Jaccard index between our dMRI tractography strategy and the histological DRTT is above 0.7. In the future, other tractography strategies can be tested using this framework.
INTRODUCTION:
Tremors, a prevalent feature of essential tremor and Parkinson’s disease, can be controlled by altering activity of the ventral intermediate nucleus (Vim) via deep brain stimulation surgery1-3 or stereotactic radiosurgery4-7. As the major fiber tract afferent to the Vim, the dentato-rubro-thalamic-tract (DRTT8) has recently been suggested as a target for tremor control9-12, however, its efficacy has been challenged13 because different approaches to diffusion-based tractography exhibited significantly different sensitivities in detecting the DRTT14. Our study aims to quantitatively evaluate the performance of existing tracking strategies by comparison to DRTT histology in the same squirrel monkey brains.METHODS:
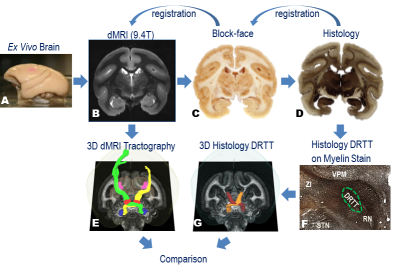
The pipeline of the method is briefly summarized in Fig. 1.
Diffusion MRI (dMRI)
The fixed brain was scanned on a 9.4T magnet using a 3D spin-echo diffusion-weighted sequence (TE/TR=41ms/410ms, 0.3mm isotropic voxels, b=3000s/mm2, 100 diffusion gradient directions, total scan time>28 hours). Probabilistic tractography was performed using FSL tools15. The seed region for tractography was the dentate nucleus, which was easily segmented on the FA map in deep cerebellum. The waypoint region was the contralateral red nucleus, which was identified on b0 images16. The other tractography parameters were roughly optimized (step size = 0.1mm, minimum length = 20mm and maximum length = 100mm). The output fiber density map was thresholded at 10% of the maximum density. Left and right DRTT were traced and thresholded respectively.
Histology
After scanning, the fixed brain was sectioned coronally at 50μm thickness using a microtome. The tissue block face was photographed after every 3rd section to assist in registration. The tissue sections were divided into six series. Series 1 was stained for myelin using the Gallyas method. Series 2 was processed with Nissl to show cytoarchitecture. The stained sections were automatically photographed using a Leica SCN400 slide scanner at 0.5μm resolution. An expert outlined the DRTT on the high resolution myelin stain micrographs using existing atlases17, 18 as references. Then the drawings were digitalized, downsampled to 150μm and then transformed into dMRI space using nonlinear registration19, 20.
Comparison of dMRI with histology
We rendered both dMRI tractography and histological DRTT in dMRI space for visual comparison. To quantitatively evaluate the agreement of tractography with histology, we calculated the Jaccard index, defined as the size of intersection of tractography and histology divided by the size of the union of the two. The region we compared was the volume between the dentate nucleus (DN) in cerebellar and ventral lateral nucleus primary division (VLp) in contralateral thalamus.
RESULTS:
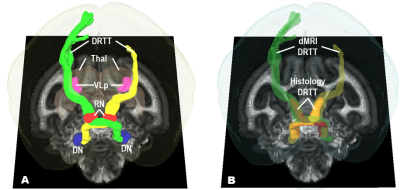
Figure 2 shows the dMRI tractography and histological DRTT in the same dMRI space. The DRTT tracts started from the DN in cerebellum, crossed over its decussation, passed through the contralateral red nucleus (RN) and then VLp (homologous to the Vim in human21) in thalamus (Thal), and eventually rose into contralateral motor cortex, as shown in Fig. 2A. Myelin data reveal the DRTT pathway from DN to VLp, as shown in Fig 2B. The Jaccard index was 0.70 and 0.74 for the left and right DRTT.DISCUSSION:
Our tractography result in the monkey brain is homologous to the DRTT in the human brain described in previous studies8, 16, which suggests the squirrel monkey can be an effective surrogate to investigate challenges in identifying the anatomical DRTT in the human brain. A limitation of this work is that myelin is not a tract-specific stain, leading to an overestimation of the DRTT volume. Meanwhile, myelin is not able to reveal the tracts above VLp. Therefore, in future work a neurotracer (e.g. biotinylated dextran amine) will be added into experiments to display a more accurate and complete DRTT.CONCLUSION:
We compared the dMRI tractography-derived DRTT and histology DRTT. The agreement between the two is high, which implies the tractography strategy we tested is effective in measuring the true DRTT in vivo. Our work also provides a framework for testing other tractography strategies, especially those commonly used in planning surgery to treat tremor patients.Acknowledgements
The study is supported by the NIH grant R01 NS058639 and a Vanderbilt Institute for Surgery and Engineering (VISE) pilot grant. We also thank the Vanderbilt Advanced Computing Center for Research and Education (ACCRE) and Vanderbilt Digital Histology Shared Resource (DHSR).References
1. Benabid AL, Pollak P, Hoffmann D, et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. The Lancet. 1991/02/16/ 1991;337(8738):403-406.
2. Rodriguez-Oroz MC, Obeso JA, Lang AE, et al. Bilateral deep brain stimulation in Parkinson's disease: a multicentre study with 4 years follow-up. Brain. 2005;128(10):2240-2249.
3. Deuschl G, Schade-Brittinger C, Krack P, et al. A Randomized Trial of Deep-Brain Stimulation for Parkinson's Disease. New England Journal of Medicine. 2006/08/31 2006;355(9):896-908.
4. Franzini A, Marchetti M, Brait L, et al. Deep brain stimulation and frameless stereotactic radiosurgery in the treatment of bilateral parkinsonian tremor: target selection and case report of two patients. Acta Neurochirurgica. 2011/05/01 2011;153(5):1069-1075.
5. Luo G, Neimat JS, Cmelak AJ, et al. Margin of Error for a Frameless Image Guided Radiosurgery System: Direct Confirmation Based on Posttreatment MRI Scans. International Journal of Radiation Oncology • Biology • Physics. 2015;93(3):S84.
6. Duma CM, Jacques DB, Kopyov OV, Mark RJ, Copcutt B, Farokhi HK. Gamma knife radiosurgery for thalamotomy in parkinsonian tremor: a five-year experience. Journal of Neurosurgery. 1998/06/01 1998;88(6):1044-1049.
7. Young RF, Shumway-Cook A, Vermeulen SS, et al. Gamma knife radiosurgery as a lesioning technique in movement disorder surgery. Journal of Neurosurgery. 1998/08/01 1998;89(2):183-193.
8. Gallay MN, Jeanmonod D, Liu J, Morel A. Human pallidothalamic and cerebellothalamic tracts: anatomical basis for functional stereotactic neurosurgery. Brain Structure & Function. 2008;212(6):443-463.
9. Coenen VA, Allert N, Mädler B. A role of diffusion tensor imaging fiber tracking in deep brain stimulation surgery: DBS of the dentato-rubro-thalamic tract (drt) for the treatment of therapy-refractory tremor. Acta Neurochirurgica. 2011// 2011;153(8):1579-1585.
10. Coenen VA, Allert N, Paus S, Kronenbürger M, Urbach H, Mädler B. Modulation of the Cerebello-Thalamo-Cortical Network in Thalamic Deep Brain Stimulation for Tremor: A Diffusion Tensor Imaging Study. Neurosurgery. 2014;75(6).
11. Kim W, Sharim J, Tenn S, et al. Diffusion tractography imaging–guided frameless linear accelerator stereotactic radiosurgical thalamotomy for tremor: case report. Journal of Neurosurgery. 2017:1-7.
12. Fenoy AJ, Schiess MC. Deep Brain Stimulation of the Dentato-Rubro-Thalamic Tract: Outcomes of Direct Targeting for Tremor. Neuromodulation: Technology at the Neural Interface. 2017;20(5):429-436.
13. Schlaier J, Anthofer J, Steib K, et al. Deep Brain Stimulation for Essential Tremor: Targeting the Dentato-Rubro-Thalamic Tract? Neuromodulation: Technology at the Neural Interface. 2015;18(2):105-112.
14. Schlaier JR, Beer AL, Faltermeier R, et al. Probabilistic vs. deterministic fiber tracking and the influence of different seed regions to delineate cerebellar-thalamic fibers in deep brain stimulation. European Journal of Neuroscience. 2017;45(12):1623-1633.
15. Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. Jan 2007;34(1):144-155.
16. Kwon HG, Hong JH, Hong CP, Lee DH, Ahn SH, Jang SH. Dentatorubrothalamic tract in human brain: diffusion tensor tractography study. Neuroradiology. 2011/10/01 2011;53(10):787-791.
17. Gergen JA, MacLean PD. A Stereotaxic Atlas of the Squirrel Monkey's Brain (Saimiri sciureus): U.S. Department of Health, Education, and Welfare; 1962.
18. Emmers R, Akert K. Stereotaxic Atlas of the Brain of the Squirrel Monkey: University of Wisconsin Press; 1963.
19. Rohde GK, Aldroubi A, Dawant BM. The adaptive bases algorithm for intensity-based nonrigid image registration. IEEE Transactions on Medical Imaging. 2003;22(11):1470-1479.
20. Choe A, Gao Y, Li X, Compton K, Stepniewska I, Anderson A. Accuracy of image registration between MRI and light microscopy in the ex vivo brain. Magnetic Resonance Imaging. 2011;29(5):683-692.
21. Stepniewska I, Preuss TM, Kaas JH. Architectonic subdivisions of the motor thalamus of owl monkeys: Nissl, acetylcholinesterase, and cytochrome oxidase patterns. The Journal of Comparative Neurology. 1994;349(4):536-557.
Figures