0735
The existence of the inferior fronto-occipital fasciculus (IFOF) revealed in the non-human primate by ex-vivo diffusion-weighted tractography and blunt dissection.1Groupe d’Imagerie Neurofonctionnelle, Institut des Maladies Neurodégénératives (GIN-IMN) - UMR 5293, CNRS, CEA Université de Bordeaux, Bordeaux, France, 2Division of Neurosurgery, Structural and Functional Connectivity Lab, Azienda Provinciale per i Servizi Sanitari (APSS), Trento, Italy, 3Neurosurgery Unit, Department of Neuroscience and Neurorehabilitation, Bambino Gesù Children Hospital, IRCCS, Rome, Italy, 4École d'optometrie, Université de Montréal, Montreal, QC, Canada, 5Laboratory of Neuropsychiatry and Psychiatric Centre Copenhagen, University of Copenhagen, Copenhagen, Denmark, 6Danish Research Centre for Magnetic Resonance, Center for Functional and Diagnostic Imaging and Research, Copenhagen University Hospital Hvidovre, Hvidovre, Denmark, 7Department of Applied Mathematics and Computer Science, Technical University of Denmark, Kongens Lyngby, Denmark
Synopsis
The existence of a ventral fronto-occipital association pathway in non-human primates similar to the inferior fronto-occipital fasciculus (IFOF) in humans, is nowadays still largely debated. In this study, we elucidate the existence, course and terminations of such a pathway using in the same non-human primate (vervet monkey) both ex-vivo diffusion-weighted tractography and blunt microdissection. From a methodological point of view, it allows an unprecedented anatomical validation of advanced tractography with microdissection for the first time in the same specimen.
Purpose
Up to now, classical autoradiographic histological tract tracing and diffusion-weighted tractography1 did not provide evidence in support of the existence of a ventral fronto-occipital association pathway in monkey similar to the inferior fronto-occipital fasciculus (IFOF) in humans2-5. Here we used both microdissection and tractography in the same specimen to elucidate the existence of the IFOF in non-human brain.Methods
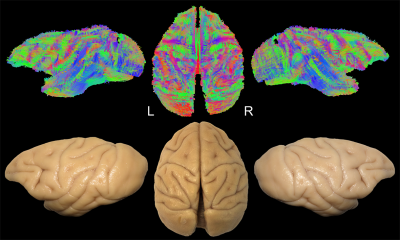
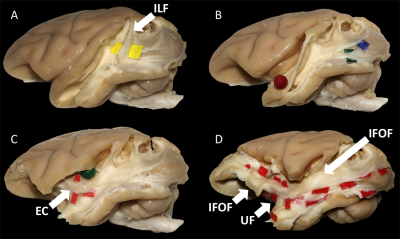
Diffusion-weighted images of 2 fixated vervet monkey brains (Chlorocebus pygerythrus) were acquired ex-vivo using a 7T-Bruker scanner (128 directions, b=7700 s/mm2, 500 µm isotropic resolution) as described previously6. Whole-brain tractograms (Fig-1) were computed with a constrained spherical deconvolution particle-filter tracking algorithm (CSD-PFT, 100 seeds per voxels in the gray/white matter interface, step 0.1, theta angle 60°)7. Bundle extraction was then performed using TrackVis8 with regions of interest (ROI) manually positioned on color coded fractional anisotropy (FA) maps guided by existing stem-based IFOF extraction in humans5. Specimens were arranged for blunt microdissection (Fig-1) with the Klingler preparation9. The dissection started in each hemisphere with the removal of the cortical gray matter (GM) along the different sulci highlighting the U-fibers. The inferior longitudinal fascicle (ILF) was first exposed (Fig-2A), running inferiorly and anteriorly from the occipital cortex. Then, we exposed a layer of horizontal fibers that spreads posteriorly to the whole occipital lobe (Fig-2B). Removing the insula GM exposed the fibers of the external capsule (EC) arching between the frontal and temporal opercula (Fig-2C). Thereafter, these were partially cut to expose the ventral third of the claustrum and EC. Removing the residual claustrum GM revealed a thick stem running posteriorly within the temporal lobe and anteriorly fanning out in the ventral frontal cortex. The fibers of the uncinate fascicle (UF) arching to the temporal pole were separated (Fig-2D), which exposed the horizontal fibers coming from the occipital lobe and projecting to the ventral part of the frontal cortex. All these steps of dissection were captured with a high-definition camera and pictures were stored for the off-line analysis of course, terminations and anatomical relationships of the bundles dissected.Results and Discussion
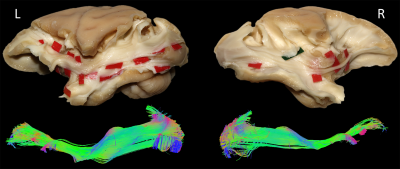
In each of the four hemispheres, both microdissection and tractography allowed to expose the same bundle of fibers connecting the whole occipital lobe with the ventral frontal cortex (Fig-3). These ventral fronto-occipital fibers are clearly distinct from the ILF that showed an infero-anterior directed course, from the dorsal and lateral occipital cortices to the anterior and basal temporal lobe confirming previous histological ILF data in monkey10. Both microdissection and tractography revealed that these fibers run anteriorly in the depth of the WM of temporal lobe, laterally to the optic radiations, and form a narrow stem at the ventral third of the external capsule (Fig-3, top). Interestingly, the overall shape of this bundle and its stem was similar to the human IFOF2-5, but also to the one recently described in the marmoset monkey using in-vivo tractography11. The present IFOF was located dorsally and posterior in respect to the uncinate fascicle (UF) stem (Fig-2D) and also clearly distinct from the extreme capsule (EC). Note that, like in humans, monkey EC fibers run in a more superficial layer (Fig-2C) with a complete different direction arching between the fronto-opercular and temporo-opercular cortices1,9. This first-time microdissection performed in non-human primate allows for the undisputable demonstration of the existence of a ventral fronto-occipital pathway as a validation for the IFOF evidenced with tractography. It cannot result from conflation of the posterior ILF with the anterior EC and UF which, as previously suggested10, may produce a spurious apparent continuum of fronto-occipital fibers. Finally, we observed that these long-range fronto-occipital fibers stop their course in the ventro-lateral, orbital and polar cortices of the frontal lobe (Fig-3).Conclusion
By comparing tractography and blunt microdissection in the same specimen, we demonstrated the existence of the IFOF in vervet monkey that showed a structural organization similar to the human IFOF. Therefore, as in humans, the anatomical framework connected by IFOF fibers may subserve the elaboration of visual information for conceptualization aimed to structure motor actions and re-actions and for basic communication acts. It shed a new light in the anatomical evolution of the ventral pathway from non-human to human primates, demonstrating a missing link for the comprehension of evolution of language and social behavior in a brain system older of tens millions of years old.
From a methodological point of view, it allows an unprecedented anatomical validation of advanced tractography by demonstrating that, when extracted in the same specimen, both virtual IFOF obtained in tractography and dissected IFOF match perfectly well.
Acknowledgements
No acknowledgement found.References
1. J. D. Schmahmann, D. N. Pandya, R. Wang et al., Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain 130, 630-653 2007.
2. J. Martino, C. Brogna, S. G. Robles et al., Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex 46, 691-699 2010.
3. S. Sarubbo, A. De Benedictis, I. L. Maldonado et al., Frontal terminations for the inferior fronto-occipital fascicle: anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain structure & function 218, 21-37 2013.
4. E. Caverzasi, N. Papinutto, B. Amirbekian et al., Q-Ball of Inferior Fronto-Occipital Fasciculus and Beyond. PLoS ONE 9, e100274 2014.
5. J. Hau, S. Sarubbo, G. Perchey et al., Cortical terminations of the inferior fronto-occipital and uncinate fasciculi: Stem-based anatomical virtual dissection Front Neuroanat 10, 58 2016.
6. T. B. Dyrby, W. F. C. Baaré, D. C. Alexander et al., An ex vivo imaging pipeline for producing high-quality and high-resolution diffusion-weighted imaging datasets. Human Brain Mapping 32, 544-563 2011.
7. G. Girard, K. Whittingstall, R. Deriche et al., Towars quantitative connectivity analysis: Reducing tractography biaises. NeuroImage 98, 266-278 2014.
8. R. Wang, T. Benner, A. G. Sorensen et al., in ISMRM. (Proc. Intl. Soc. Mag. Reson. Med., Berlin, 2007), vol. 15, pp. 3720.
9. S. Sarubbo, A. De Benedictis, S. Merler et al., Structural and functional integration between dorsal and ventral language streams as revealed by blunt dissection and direct electrical stimulation. Hum Brain Mapp 37, 3858-3872 2016.
10. J. D. Schmahmann, D. N. Pandya, Fiber pathways of the brain. (Oxford University Press, New York, 2006).
11. D. J. Schaeffer, R. Adam, K. M. Gilbert et al., Diffusion weighted tractography in the common marmoset monkey at 9.4 T. J Neurophysiol, 2017.
Figures